How to Cite | Publication History | PlumX Article Matrix
Varaprasad Bobbarala2, Varahala Rao Vadlapudi1 and K. Chandrasekhar Naidu1
1Department of Botany, Andhra University, Visakhapatnam India.
2For U Biosciences, A/4A, Park lane Residency, East Point Colony, Visakhapatnam - 530 017 India.
Corresponding Author E- mail: varaprasadphd@rediffmail.com
ABSTRACT: The antimicrobial activity of Thespesia populneoides plant extracts was evaluated with important microorganisms belong to plants, humans and aquatic origin. In evaluating antimicrobial activity of T. populneoides hexane, chloroform and methanol crude extracts were used. Agar cup plate method/well diffusion method was used to study the antimicrobial activity of all these crude extracts. Aeromonas hydrophila, Pseudomonas marginales, Penicellium expansum and Ustilago maydis were the most resistant bacteria and fungi. Methanolic crude extracts of T. populneoides showed strong activity against the tested microbial strains. Therefore, this can be selected for further investigation to determine its therapeutic potential.
KEYWORDS: Thespesia Populneoides; Plant crude extracts activity; Medicinal plants; Antimicrobial activity
Download this article as:| Copy the following to cite this article: Bobbarala V, Vadlapudi V. R, Naidu K. C. In Vitro Antimicrobial Properties of Mangrove Plant Thespesia Populneoides Against Selected Clinical, Plant and Aquatic Pathogens. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Bobbarala V, Vadlapudi V. R, Naidu K. C. In Vitro Antimicrobial Properties of Mangrove Plant Thespesia Populneoides Against Selected Clinical, Plant and Aquatic Pathogens. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=8420 |
Introduction
The problem of microbial resistance is growing and the outlook for the use of antimicrobial drugs in the future is still uncertain. Therefore, actions must be taken to reduce this problem, for example, to control the use of antibiotic, develop research to better understand the genetic mechanisms of resistance, and to continue studies to develop new drugs, either synthetic or natural. The ultimate goal is to offer appropriate and efficient antimicrobial drugs to the patient. For a long period of time, plants have been a valuable source of natural products for maintaining human health, especially in the last decade, with more intensive studies for natural therapies. According to World Health Organization medicinal plants would be the best source to obtain a variety of drugs. About 80% of individuals from developed countries use traditional medicine, which has compounds derived from medicinal plants. Therefore, such plants should be investigated to better understand their properties, safety and efficiency1. The use of plant extracts and phytochemicals, both with known antimicrobial properties, can be of great significance in therapeutic treatments. In the last few years, a number of studies have been conducted in different countries to prove such efficiency2, 3. The antimicrobial properties of plants have been investigated by a number of researchers in India. Many plants have been used because of their antimicrobial traits, which are due to compounds synthesized in the secondary metabolism of the plant. Mangroves have long been a source of astonishment for the layman and of interest for scientist and also play an important role in the suppression of deleterious microorganisms4, 5. These plant products are known by their active substances, for example, the phenolic compounds which are part of the essential oils7, as well as in tannin8. Among one is T. populneoides belongs has been using in treatment of scabies. Presently looking for more detailed study on antimicrobial compounds and evaluating their activity on plant clinical and aquatic pathogens.
Materials and Methods
populneoides, mangrove tree classified in the plant family malvaceae and vernacular name is attakanarai growing in the saline intertidal zones of sheltered coast lines Grey mangrove is a highly variable tree, with a number of ecotypes, and forms closely resembling other species. It has been reported to tolerate extreme weather conditions, high winds. The material was taxonomically identified and the Voucher specimen is stored. The plant parts were collected from Coringa Mangrove Wetland, Andhra Pradesh, India. The plant material were dried under shade with occasional shifting and then powdered with a mechanical grinder and stored in an airtight container. The powder obtained was subjected to successive soxhlet extraction with the organic solvents with increasing order of polarity respectively.
The microorganisms studied (Including fungi and bacteria) were obtained from Microbial Type Culture Collection (MTCC), Chandigarh. These organisms include Acremonium strictum (MTCC 2599), Aeromonas hydrophila (MTCC 646), Aspergillus flavus (MTCC 4633), Asperigillus niger (MTCC 2723), Bipolaris bicolor (MTCC 2105), Candida albicans (MTCC 3017), Cladosporium herbarum (MTCC 21430), Erwinia carotovora (MTCC 3609), Fusarium moniliforme, Fusarium oxysporum (MTCC 1755), Lactobacillus acidophilus (MTCC 447), Macrophomina phaseolina (MTCC 2165), Pantoea agglomerans (MTCC 2959), Penicellium expansum (MTCC 2006), Pseudomaonas marginales (MTCC 2758), Pseudomonas spp., Staphylococcus aureus (MTCC 96), Streptococcus mitis (MTCC 2696), Streptococcus mutans (MTCC 890), Streptococcus salivarius (MTCC 1938), Ustilago maydis (MTCC 1474) and Xanthomonas compestris (MTCC 2286). The strains are maintained and tested on Nutrient Agar (NA) for bacteria and Potato Dextrose Agar (PDA) for fungi. Active cultures were generated by inoculating a loop full of culture in separate 100mL nutrient broths and incubating on a shaker at 37oC overnight. The cells were harvested by centrifuging at 4000 rpm for 5 min, washed with normal saline, spun at 4000 rpm for 5 min again and diluted in normal saline to obtain 5 x 105cfu/mL.
Determination of antibacterial activity
The crude extracts of the different plant parts of different species were subjected to antimicrobial assay using the agar well diffusion method of Murray et al., 9 modified by Olurinola10. 20 ml of nutrient agar was dispensed into sterile universal bottles these were then inoculated with 0.2 ml of cultures mixed gently and poured into sterile petri dishes. After setting a number 3-cup borer (6mm) diameter was properly sterilized by flaming and used to make three to five uniform cups/wells in each petri dish. A drop of molten nutrient agar was used to seal the base of each cup. The cups/wells were filled with 50µℓ of the extract concentration of 100mg/ml and allow diffusing for 45 minutes. The solvents used for reconstituting the extracts were similarly analyzed. The plates were incubated at 37°c for 24 hours for bacteria. The above procedure is allowed for fungal assays but except the media potato dextrose agar instead of nutrient agar and incubates at 25°c for 48 hours. The zones of inhibition were measured with antibiotic zone scale in mm and the experiment was carried out in duplicates. The extracts and the phytochemicals that showed antimicrobial activity were later tested to determine the Minimal Inhibitory Concentration (MIC) for each bacterial and fungal sample.
Results and Discussion
The data pertaining to the antimicrobial potential of the plant extracts presented in Tables 1 and 2, respectively. The extracts obtained from chloroform and methanol presented antimicrobial activity to at least one of the tested microorganisms. The extracts of methanol presented the highest activities. On the other hand, the extracts from hexane did not show any anti-microbial activity. One of the microorganisms that showed susceptibility to these extracts was Erwinia carotovora and Cladosporium herbarum. The microorganism Aeromonas hydrophil, Penicellium expansum and Ustilago maydis were resistant to the plant extracts tested. The data obtained, through the determination of MIC, from the crude extracts of chloroform and methanols are presented in Table 2. The results revealed variability in the inhibitory concentrations of each extract for given microorganism. The methanolic extracts showed activities in the range (concentrations) from 10 to 100mg/mL.
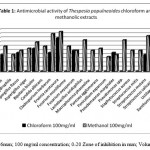 |
Table 1
|
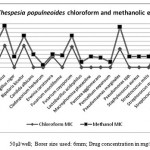 |
Table 2
|
The use of plants to control diseases has been extensively studied by people. Data obtained from the literature as well as our research findings reveal the great potential of T. populneoides plant extracts for therapeutic treatment, in spite of the fact that they have not been completely investigated. Therefore, more studies need to be conducted to search for new antimicrobial compounds. Once extracted, and before being used in new therapeutic treatments, they should have their toxicity tested in vivo. Bioassays11, 12 have demonstrated the toxicity of extracts from different plants. Therefore, our results revealed the importance of plant extracts to control resistant bacteria, which are becoming a threat to human health. Furthermore, in a few cases, these plant extracts were active against antibiotic resistant bacteria under very low concentration, thus minimizing the possible toxic effects.
Borer size used: 6mm; 100 mg/ml concentration; 0-20 Zone of inhibition in mm; Volume per well: 50μl, 50μl/well; Borer size used: 6mm; Drug concentration in mg/ml;
References
- Ellof J. N., Ethnopharmacol., 60: 1-6 (1998).
- Ikram M. and Inamul H., Fitoterapia 55: 62-64 (1984).
- Kubo L., Muroi H. and Himejima M., Agri. Food Chem. 41: 1016-1019 (1993).
- Jamale B. B. and G. V. Joshi., J. Exp. Biol., 16(1): 117-120 (1998).
- Nishiyama Y., Ryuzo P.C., Sanchez and M. Kozaki., Hakko, Kogaku, Kaishi, 56: 712-717 (1978).
- Ross S. A., Megalla S. E., Bisby D. W. and A. H. Awad., Fitoterpia, 51: 303-308 (1980).
- Jansen A. M., Cheffer J. J. C. and Svendsen., Planta Med. 40: 395-398 (1987).
- Saxena G., McCutcheon A.R., Farmer S. and Towers G.H.N., Ethnopharmacol. 42: 95-99 (1994).
- Murray P. R., Baron E. J., P faller M. A., Tenover F. C. and Yolken H. R., Manual of clinical microbiology, 6th Edition, ASM Press, Washington DC, 15 (1995).
- Olurinola P. F., A laboratory manual of pharmaceutical microbiology. Printed by National Institute for Pharmaceutical Research and Development, Idu, Abuja, Nigeria, 69 (1996).
- Carvalho V., Melo V. M., Aguiar A. and Matos F. S., Ciencia e Cultura 40: 1109-1111 (1988).
- Nascimento S.C., Chiappeta A. and Lima, R.M.O.C., Fitoterapia 61: 353-355 (1990).

This work is licensed under a Creative Commons Attribution 4.0 International License.





