How to Cite | Publication History | PlumX Article Matrix
Abirami1, V. Shanmugaraju², A. Mahalakshmipriya² and K. Rajathi¹
¹Department of Biochemistry, Dr. N.G.P. Arts and Science College. KMCH Research and Educational Institutions, Kalappati Road, Coimbatroe - 48 India.
²Department of Biotechnology, Dr. N.G.P. Arts and Science College. KMCH Research and Educational Institutions, Kalappati Road, Coimbatroe - 48 India.
ABSTRACT: The present study was carried out to evaluate the in vitro hepatoprotective activity of 90% ethanolic and aqueous extracts of leafs of Asteracantha longifolia and Andrographis paniculata against lead acetate induced hepatic damage. In addition, antioxidant activity was also studied in the aqueous and ethanolic leaf extracts of Asteracantha longifolia and Andrographis paniculata. The hepatoprotective property of aqueous and ethanolic extracts of Asteracantha longifolia and Andrographis paniculata was investigated by using lead acetate to induce toxicity. The extent of lipid peroxidation (LPO), Superoxide dismutase (SOD), Catalase (CAT), Aspartate transaminase (AST), Alanine transaminase (ALT, Acid phosphatase (ACP), Alkaline phosphatase (ALP) and Lactate dehydrogenase (LDH) were assayed in the liver homogenate. In addition the antioxidant property of aqueous and ethanolic extract was checked by assaying the activities of enzymatic antioxidants and free radical scavenging activity. The activities of liver marker enzymes Aspartate transaminase, Alanine transaminase, Acid phospatase, Alkaline phosphatase, Lactate dehydrogenase and the levels of lipid peroxidation was found to be increased during the lead acetate toxicity. Administration of aqueous and ethanolic leaf extracts of Asteracantha longifolia and Andrographis paniculata to the lead acetate induced hepatotoxicity reaches to near normal. In addition, the aqueous and ethanolic leaf extracts of Asteracantha Longifolia and Andrographis paniculata have potent antioxidant effects against several antioxidants/ROS and capability to induce the invitro antioxidant system. The results indicate that the leafs of Asteracantha longifolia and Andrographis paniculata possess hepatoprotective property possibly because of its antioxidant activity.
KEYWORDS: Asteracantha longifolia; Andrographis paniculata; In vitro hepatoprotective; antioxidant; lead acetate toxicity
Download this article as:| Copy the following to cite this article: Abirami N, Shanmugaraju V, Mahalakshmipriya A, Rajathi K. In Vitro Hepatoprotective and Antioxidant Activities of the Leaf Ethanolic and Aqueous Extracts of Asteracantha Longifolia and Andrographis Paniculata Against Lead Acetate Induced Toxicity. Biosci Biotechnol Res Asia 2009;6(1). |
| Copy the following to cite this URL: Abirami N, Shanmugaraju V, Mahalakshmipriya A, Rajathi K. In Vitro Hepatoprotective and Antioxidant Activities of the Leaf Ethanolic and Aqueous Extracts of Asteracantha Longifolia and Andrographis Paniculata Against Lead Acetate Induced Toxicity. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=20111 |
Introduction
Recent times natural products derived from plants are focused in search of new drugs by indicating new modes of pharmacological action. However there is a lack of precise guidelines to study the herbal compunds and till date a very meagre portion of this tremendous potential drug-repertoire has been sientifically screened. Hence there is a real need for scientific based validation of these agents[1]. By viewing the scope and significance of the plant based medicine the present study has been focused to investingate the medicinal potentiality of the plants that belongs to the family acanthaceae.
Andrographis paniculata (family: Acanthaceae) is a much branched erect annual herb. Branches are four angled. Leaves are lance- shaped. Flowers appear in large spreading sparse branches. The constituents found in plants are kalmeghin and andrographolide. The herb is reported to possess astringent, anodyne, antipyretic and useful in dysenter y, cholera, diabetes, bronchitis, swellings and itches, piles and gonorrhea12.
Asteracantha longifolia (family: Acanthaceae) is a robust, erect, annual herb. The stems are sub-quadrangular with thickened nodes. The leaves are oblanceolate, with a yellow spine in its axil. The flowers are pale, purple blue, densely clustered in axils. The fruits are oblong, glabrous capsules, 4-8 seeded. It grows throughout India. The chemical composition include alkaloids, phytosterol, mucilage and potassium salts. The roots, leaves and seeds have been used in Indian systems of medicine as diuretics and also employed to cure jaundice, dropsy, rheumatism, anasarca and diseases of the urinogenital tract [3,4]. However the antioxidant and invitro hepatoprotective effect of aqueous and ethanolic extracts of Asteracantha longifolia and Andrographis paniculata against lead acetate (1mm/g) induced toxicity in tissues remains unexplore. Therefore the present study was undertaken to evaluate antioxidant and protective and preventive role against lead induced toxicity.
Material and Methods
Plant material
The whole plant of Asteracantha longifolia and Andrographis paniculata were collected from Tamilnadu Agricultural University and Institute of Forest Genetics and Tree Breeding, Coimbatore.
Preparation of extracts
The leafs of the plants were taken and shade dried and made in to a coarse powder. About 25g of coarse powder was subjected to successive soxhlet extraction using 90% ethanol and distilled water according to the order of polarity for 48 hours. The solvent was removed under reduced pressure which gave a greenish black coloured sticky residue.
Antioxidant activity
Free radical Scavenging Activity
Super oxide scavenging activities of compounds were determined by the method of Liu and Chang (1997)5, which depends on the light, induced super oxide generation by riboflavin and the corresponding reduction of Nitro blue terazoluim (NBT).
Hydroxyl radical scavenging activity was measured by studying the competition between deoxy ribose and test compounds for hydroxyl radicals generated from the Fe 3+/ascorbate/EDTA/ H O System of Halliwell et al., 19876.
Nitric oxide was generated from sodium nitroprusside and measured by Griess reaction. DPPH Radical Scavenging Activity was measured according to the method of Shimada etal., 1992[7]. Ferric reducing ability of the plant as measuring of antioxidant power was measurd. Hydrogen Peroxide-Scavenging Activity was determined by the method of Ruch et al., 19898.
The antioxidant activity of the extracts was evaluated using the β- Carotene-Linoleate Model System (Miller, 1971) [9].
Enzymatic antioxidants
Activity of catalase was determined by the method of Luck, 1974. Peroxidase activity was determined by the method of Reddy etal., 1995. Polyphenoloxidase was estimated by the method of Esterbaur et al.,1997.
Evaluation of hepatoprotective activity of plant leaf extracts
About 2.0g of goat liver was weighed and incubated with lead acetate (1.0mM/g) for one hour to induce toxicity.
Experimental Set Up
Group I : Served as control
Group II : Toxicity (Administered lead
acetate 1.0mM/g for one hour and Incubated).
Group III : Curative
´ Lead Toxicity + Aqueous extracts of
Asteracantha longifolia
´ Lead Toxicity + Ethanolic extracts of
Asteracantha longifolia
´ Lead Toxicity + Aqueous extracts of
Andrographis paniculata
´ Lead Toxicity + Ethanolic extracts of
Andrographis paniculata
Group IV : Preventive
´ Aqueous extracts of Asteracantha longifolia.
´ Ethanolic extracts of Asteracantha longifolia.
Table 1: Free radical scavenging activity of plant leaf extracts.
| S. | Parameters | Asteracantha | Longifolia | Andrographis | Paniculata |
| No. | Aqueous | Ethanol | Aqueous | Ethanol | |
| Extract | Extract | Extract | Extract | ||
| 1 | DPPH | 42.69 | 34.59 | 54.98 | 77.44 |
| 2 | FRAP | 400 | 376 | 475 | 505 |
| 3 | Scavenging of Nitric Oxide | 41.82 | 74.21 | 39.98 | 75.27 |
| radical | |||||
| 4 | Scavenging of | 76.32 | 38.13 | 77.83 | 47.17 |
| hydroxyl radical | |||||
| 5 | Scavenging of | 26.49 | 32.85 | 31.09 | 39.98 |
| Hydrogenperoxide radical | |||||
| 6 | Scavenging of | 41.82 | 40.81 | 51.07 | 49.97 |
| Super oxide radical | |||||
| 7 | B carotene- | 54.76 | 47.62 | 47.62 | 57.14 |
| linoleic acid assay |
Units of free radical scavenging activities are expressed in % of radical scavenged for (DPPH, Nitricoxide radical, Hydroxyl radical, Hydrogen peroxide radical, superoxide radical, β- carotene linoleic acid assay.
FRAP is expressed in units of um.
Table 2: Enzymatic Antioxidant Activity of Plant Leaf Extracts
S. Enzymatic Asteracantha Longifolia Andrographis Paniculata
| No. | antioxidants | Aqueous Extract | Ethanol Extract | Aqueous Extract | Ethanol Extract | |
| 1. | Catalase | 39.4 | 32.93 | 34.47 | 52.25 | |
| 2 | Polyphenol | Catechol | 1.414×10-3 | 0.6528×10-3 | 1.694×10-3 | 1.305×10-3 |
|
3 |
oxidase Peroxidase | Laccase | 1.258×10-3
24.5 |
0.5808×10-3
12.0 |
1.904×10-3
22.01 |
1.162×10-3
13.0 |
Units are expressed in %. Values of Mean ± SD.
Treatment of groups are as follows:
Group I (Control), Group II (Tocicity), Group III (Curative), Group IV (Preventive).
Aqueous extract of Asteracantha longif
Ethanol extract of Asteracantha longif
Aqueous extract of Andrographis paniculata.
Ethanol extract of Andrographis
Enzyme Units are expressed as: ACP – Micromole of phenol; ALP – Micro molar of phenol, LDH – Micro moles of pyruvate, Aminotransferase-micro moles of pyruvate liberated/min/mg protein at 370C.
Comparisons are made between groups are as follows:
Between group I and group II
Between group II and group III
Between group I and IV.
The symbols represent the statistical significance: * p <0.001 $ pNS
´ Aqueous extracts of Andrographis paniculata.
´ Ethanolic extracts of Andrographis paniculata.
At the end of the experimental setup liver was removed and washed with saline two to three times and the liver homogenate was prepared for further enzymatic analysis.
Preparation of Liver Homogenate
A 10% homogenate of the liver tissues were prepared in 0.01M Tris HC1 buffer, pH 7.4 using Teflon homogenizer. The homogenate was centr ifuged at 12000g for 30 minutes in a refrigerator high speed centrifuge. The supernatant was used for the assay of enzymes such as ACP10, AST, ALP11, LDH10, SOD12, CAT13, LPO14 and
protein15. Results of biochemical estimations were reported as mean± DF in each group. The data were subjected by student’s t test. p<0.001 was considered as statistically significant.
Results and Discussion
Antioxidant Activity
Free radical scavenging activity
The aqueous and ethanolic extract of Asteracantha longifolia and Andrographis paniculata were subjected to screening for their possible antioxidant activity. The complementar y test systems namely DPPH free radical scavenging, FRAP, β- Carotene linoleic acid system, Nitric oxide radical scavenging, Hydrogen peroxide radical scavenging system, Hydroxyl radical scavenging systems and superoxide anion scavenging system were used for the analysis of the antioxidant capacity of the aqueous and ethanolic extract extracts of Asteracantha longifolia and Andrographis Paniculata have potent antioxidant effects against ROS and have the capability to induce the invitro antioxidant systems. The results are tabulated (Table 1).
Enzymes as antioxidants
Table-2 illustrates the percentage inhibition of enzymatic antioxidants generated by aqueous and ethanolic extracts of Asteracantha longifolia and Andrographis paniculata.
Table 4: Activities of protein, SOD, Catalase, Lipid Peroxidation in the Normal and Experimental Groups
- S. Param- Group I Group II Group III (Curative) Group IV (Preventive)
No. eters (Induced Toxicity) A B C D A B C D (Control)
1 Protein 1.57±0.06 0.67±0.05a* 0.98±0.04b1* 0.89±0.08b2* 0.99±0.05b1* 1.02±0.08b1* 0.47±0.08c1$ 1.46±0.07c2$ 1.47±0.05c3$ 1.51±0.07c4$
2 SOD 03.92±0.12 1.28±0.11 a* 2.04±0.13b1* 2.42±0.14 b2* 2.51±0.13 b3* 2.41±0.17 b4* 3.79±0.1c1$ 3.83±0.12 c2$ 3.82±0.13 c3$ 3.81±0.12 c4$
3 Catalase 25.82±0.91 10.18±0.86a* 17.05±0.87 b1* 16.61±0.82 b2* 15.59±0.82 b3* 18.28±0.81 b4* 23.5±0.61 c1$ 24.10±0.62 c2$ 23.780±0.63 c3$24.120±0.64 c4$
4 LPO 2.85±0.13 4.27±0.21 a* 3.22±0.18 b1* 3.18±0.17 b2* 3.25±0.17 b3* 3.21±0.21 b4* 2.77±0.21 c1$ 2.75±0.12 c2$ 2.79±0.12 c3$ 2.75±0.12 c4$
Values of mean ± SD
Treatment of group are as follows:
Group I (Control), Group II (Toxicity), Group III (Curative), Group IV (Preventive)
Aqueous extract of Asteracantha longif
Ethanol extract of Asteracantha longif
Aqueous extract of Andrographis paniculata.
Ethanol extract of Andrographis
Enzyme Units are expressed as: SOD: Units/mg Protein, Catalase: µ moles of the H2O2 consumed/min/mg protein, LPO-Nanomoles of MDA formed / mg protein at 37 C.
Protein – mg/g tissue
Comparisons are made between groups are as follows:
Between group I and group II
Between group II and group III
Between group I and IV.
The symbols represent the statistical significance:
* p <0.001 $ pNS.
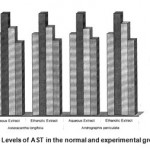 |
Figure 1: Levels of AST in the normal and experimental groups.
|
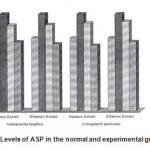 |
Figure 2: Levels of ASP in the normal and experimental groups.
|
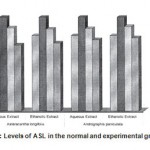 |
Figure 3: Levels of ASL in the normal and experimental groups.
|
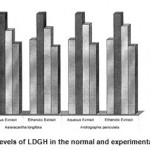 |
Figure 4: Levels of LDGH in the normal and experimental groups.
|
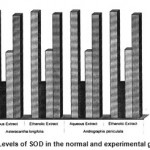 |
Figure 5: Levels of SOD in the normal and experimental groups.
|
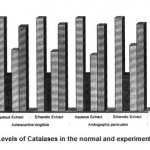 |
Figure 6: Levels of Catalases in the normal and experimental groups.
|
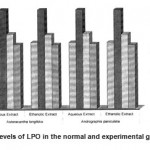 |
Figure 7: Levels of LPO in the normal and experimental groups.
|
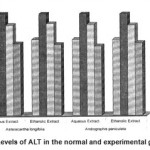 |
Figure 8: Levels of ALT in the normal and experimental groups.
|
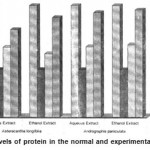 |
Figure 9: Levels of protein in the normal and experimental groups.
|
Invitro Hepatoprotective Activity
The activities of liver marker enzymes AST, ALT, ACP, ALP and LDH, and the levels of lipid peroxidation was found to be increased during the lead acetate toxicity p>0.001. The levels of antioxidant enzymes (SOD, Catalase) and protein were found to be decreased during lead acetate toxicity p>0.001. Administration of aqueous and ethanolic leaf extracts of Asteracantha longifolia and Andrographis paniculata to the lead acetate induced hepatotoxicity reaches to near normal [Table 3,4]. The statistical analysis indicates that aqueous and ethanolic leaf extracts of Asteracantha longifolia and Andrographis paniculata possess curative as well as preventive activity against lead induced toxicity t\in liver.
Endogenous antioxidant enzymes (SOD
& CAT) are responsible for preventing and neutralizing the free radical induced damages on tissues16. Lead induced decrease in SOD and CAT might be due to the formation of reactive oxygen species by lead17.
A significant increase in the levels of TBARS in animals treated with lead showed the induction of lipid peroxidation by lead. This might be due to the release of free radicals and membrane
damage by lead. Lead is reported to release free radicals there by stimulating the process of lipid peroxidation.
Lipid peroxides have been shown to impair tissue membranes which is a risk factor in variety of diseases18.
The site specific oxidative damage of some of the susceptible amino acids of proteins is now regarded as the major cause of metabolic dysfunction during pathogenesis19. The capacity of liver to synthesize albumin is adversely affected by hepatotoxins20. The lowered level of total proteins recorded in the present study can be attributed to these features.
Protective agents from plant origin with antiperoxidative and antioxidant properties play an important role in protecting the liver against toxicity21.
Conclusion
The present study demonstrates that the aqueous and ethanolic lead extracts of Asteracantha longifolia and Andrographis paniculata possess hepatoprotective property possibly because of its antioxidant activity.
References
- Jaya Kumari, Sahoo, P.K., Giri, S.S., Effects of polyherbal formulations (Immunoplus on immunity and disease resistant of India major corp, Labia rohita at different stages of growth, Indian Journal of Experimental Biology, 45: 291-298 (2007).
- Pullaiah, T., Medicinal plants, medicinal plants in , 1: 54-55 (2002).
- Panigrahi, J., Behera, , Maharana, S., Mishra, R.P., Biomolecular changes during invitro organogenesis of Asteracantha longifolia (l.) Nees-A medicinal herb., Indian journal of Experimental Biology, 45 (10):911-919 (2007).
- Mistra, T.N., Singh, S., Pandev, R.P., Constituents of Asteracantha longifolia, Fitoterapia, 72(20): 194-196 (2001).
- Liu, F., Ooi, V.C., and Chang, S.T., Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 60: 763-771(1997).
- Halliwell, J.M.C., Gutteridge and Aruoma, O,I., The deoxy ribose method: a simple test tube assay for the determination of rate constants for reaction of hydroxyl radicals, Analytical Biochemistr y., 165: 215-219 (1987).
- Shimada, , Fujikawa, K., Yahara, K., Nar/ Kamur, T., Antioxidative properties of xanthan on the autooxidation of soybean oil in cyclodextrin emulsion., Journal of Agricultural and Food chemistry., 40: 945-948 (1992) .
- Ruch, S.J., Cheng, S.J., Klawnig, J.E., 623-647 (1998).
- Prevention of cytotoxicity and inhibition of 15. Lowry, O.H. Rosenbrough, N.T. Farr, A.L. ntercellular communication by autooxidant and Randall, R.J., Protein measurementcatechins isolated from Chinese green tea., with folin phenol reagent. J. Bio. Chem., 1993; Carcinogenesis 10: 103-108 (1989). 265-275 (1951).
- Miller, H.E., A simplified method for the 16.Bandyapadhyay, D, Biswas, K, evaluation of antioxidant., Jour nal of Bandyaphadhyay, U, Reiter, R.J. andAmerican oil chemists’ society., 18: 439-452 Banerjee., Melatonin protects against stress (1971). indused gastric lesions by scavenging the
- King. E.J., The dehydrogenase or hydroxyl radical, J. Pineal Res, 29: 143-151 oxidoreductase, lactate dehydrogenase. In: (2000). practical clinical enzymology (Ed. Van, D)
- Jadhav, A.Ll, Ramesh, G.T., Gunasekar,Norstand company limited, London, 83 P.G., Toxicology lett., 115 (2): 89-98 (2000). (1965).

This work is licensed under a Creative Commons Attribution 4.0 International License.





