How to Cite | Publication History | PlumX Article Matrix
Occurrence and Characterization of Antifungal Compound in Pithophora Oedogonia
Pamela Sukumaran2 and R. Thevanathan1
1Post Graduate and Research Department of Botany, Presidency College (Autonomous) Chennai - 600 078 India.
2Department of Plant Biology and Plant Biotechnology, Women’s Christian College, Chennai - 600 006 India.
ABSTRACT: Hexane extracts of the green alga, Pithophora oedogonia was tested for antiifungal activity against isolate of a plant pathogenic fungi namely, Colletotrichum lindemuthianum . the extracts were fractionated by coloumn chromatography. By combiningTLC bioautography with coloum chromatography active fractions were identified. The compound in the active fraction was characterized by GC MS analysis. Results are presented and discussed.
KEYWORDS: Antifungal compounds; algae; Pithophora oedogonia
Download this article as:| Copy the following to cite this article: Sukumaran P, Thevanathan R. Occurrence and Characterization of Antifungal Compound in Pithophora Oedogonia. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Sukumaran P, Thevanathan R. Occurrence and Characterization of Antifungal Compound in Pithophora Oedogonia. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8111 |
Introduction
Many compounds have been isolated from alga with antifungal properties.
Codomeir et al (1977) have isolated bromine compounds that were antifungal from the algae Asparagposis armata and Falkenbergia rufolanosa. Isozonarol hydroquinone active against plant pathogenic fungi and cycloendesmol active against Candida albicans were isolated from Dictyopteris zonarioides and from Chondria oppoosicloda respectively (Fenical et al., 1973, 1974). Goniodomin, a fungicidal substance was isolated from a marine dinoflagellate Gonidoma sp. (Sharma et al., 1968). A new naphthoquinone, deoxylapachol isolated from Landsburgia quereifonlia inhibited Candida albicans and T. mentagrophytes (Perry et al., 1991). Deng– Songzhi and Deng-Sz (1997) isolated a number of novel compounds including polyethers, macrolides, terpenoids, alkaloids, peptides, sterols, polysaccharides and unsaturated fatty acids from marine algae which exhibited antifungal activity. Melo et al. (1997) isolated a protein fraction rich in agglutinins from Hypnea musciformis which showed antifungal activity against Trichophyton rubrum and Colletotrichum lindemuthianum. It could be seen that in spite of the many reports on antifungal activity of the algal extracts, very few have isolated and characterized the active compounds in the extracts. Further, the information available on the antifungal compounds derived from algae especially fresh water macroalgae against plant pathogens is negligible.
Among the fresh water algae, Spirulina and Chlorella have been extensively studied either for their nutraceutical or pharmaceutical qualities and information on these aspects is lacking for many of the fresh water algae which grow in abundance with simple growth requirements and natural conditions. With this in view Pithophora oedogonia (Mont.) Wittrock, a fresh water alga of common occurrence was investigated for its bioactive properties.
Materials and Methods
Filaments of Pithophora oedogonia were collected from cultures maintained in cement tanks containing Bold basal medium at 27 ± 2°C and a light intensity of 50 m Einsteins m-2 s-1. Harvested filaments (250 g) were shade dried and extracted with n-hexane for 48 hrs. olumn fractionations of extract residue was carried out with Silica gel G (60 – 120 mesh, 150 g, 45 x 14 cm) using hexane, 5%, 20%, 30%, 40%, 50%,60%, 80% ethyl acetate in hexane, Based on similarities on TLC, the elutes were pooled and designated as HFL 1-7. and HCC 3-6 combining TLC bioautography with coloum chromatography active fractions were identified. The compound in the active fraction was characterized by GC MS analysis.
Results
After development of the hexane extract in the appropriate solvent system, the TLC strips were dried and sprayed with a spore suspension (3,000 spores/ml) of C. lindemuthianum in Czapek Dox medium. The TLC strips were then incubated for 48 hrs and zones of inhibition were observed and compared with a reference replicate TLC strip. Clear zones of inhibition were noticed in Rf 0.8 region for the hexane extract (Plate 1). The results indicate a clear antifungal activity by the hexane extract of Pithophora oedogonia. Based on these results, further fractionation of the hexane extract was carried out on silica gel columns to isolate the antifungal fraction. Bioautography of the different column fractions of n-hexane extract residue against C. lindemuthianum showed a clear zone of inhibition at Rf 0.64 of the fraction HFL 5 (Plate I). Low inhibition zones at the Rf 0.58 of fraction HFL 2, HFL 4 and a very poor inhibition at Rf 0.41 of HCC 6 were also recorded.
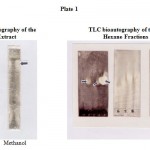 |
Plate 1
|
Gc-Ms Analysis Of The Fraction Hfl 5
The fraction HFL 5 of n-hexane extract (0.052 g) which showed activity against C. lindemuthianum was subjected to GC-MS (SHIMADZU 2p5000) analysis for characterization of the active component. The GC-MS chromatogram revealed 8 peaks in the fraction. Three peaks with retention times 19.667, 20.667 and 26.830 – 26.880 were major and considered for identification from available standards in the library (Fig. 1). The compounds were obtained by a library search (WILEY139.LIB and NIST12 LIB) available with the GC-MS System. From a hit list of standards available in the library, the 3 major peaks of HFL 5 were identified on the basis of similarity index (Fig. 2& 3 and Table 2).
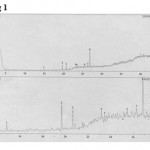 |
Figure 1
|
 |
Figure 2
|
 |
Figure 3
|
Table 2: GC-MS analysis of Pithophora oedogonia extract of HFL 5.
| Peak
No. |
Retention
Time |
Compound | SI | Mol.wt | MS data |
| 2. | 19.667 | 1,2 Benzenedicarboxylic
acid,bis (2-methylpropyl) ester |
91 | 202 | 149 (BP) 223,
205, 167 |
| 3. | 20.667 | 1,2 Benzenedicarboxylic
acid,dibutyl ester |
94 | 278 | 149 (BP) 223,
206, 160 |
| 8. | 26.830-
26.889 |
1,2 Benzenedicarboxylic
acid,dioctyl ester |
93 | 390 | 149 (BP) 167,
163 |
The GC-MS analysis of HFL 5 indicated the presence of different esters of 1,2 Benzenedicarboxylic acid. Compounds that showed less than 90% similarity index were not considered. The major component in the active fraction of n-hexane extract of Pithophora oedogonia appeared to be the esters of Phthalic acid namely isobutyl, dibutyl and dioctyl phthalate to an extent of 6%, 5% and30% respectively. Hence esters of phthalic acids were prepared and their antifungal activiy was investigated.
An authentic sample of n-butyl phthalate was prepared by esterification of phthalic acid with n-butyl alcohol in the presence of trace amounts of the acid vide Materials and Methods. The prepared sample was also subjected to GC-MS analysis and the retention time of the sample was found to be 20.858 min (Fig.4). This confirmed the presence of n-butyl phthalate in the active fraction of Pithophora oedogonia. Further, the authentic n-butyl phthalate exhibited 62.5% inhibition against
C. lindemuthianum (at 100 µg/mL concentration and 144 hours of incubation) to confirm the observation.
 |
Figure 4
|
It is the first report on the occurrence and isolation of n-butyl phthalate, an active antifungal compound from a fresh water green alga, Pithophora oedogonia. The antifungal nature of the compound is also recorded for the first time.
Discussion
TLC strips developed in the optimized solvent system showed the spot with Rf 0.8 of the n-hexane extract to contain the active component that inhibited the growth of Colletotrichum lindemuthianum. Further fractionation of the extracts was carried out by column chromatography of the dissolved extract residue on silica gel gravity column using a combination of solvent systems. Flash column chromatography was also employed wherever necessary. The fractions obtained by these column chromatographic techniques were subjected to TLC bioautography which showed the spot with Rf 0.64 of the fraction HFL 5 (Plate I) of n-hexane residue to contain the bioactive principle responsible for the inhibition of growth. The active fraction HFL 5 of n-hexane extract was subjected to GC-MS analysis for characterization of the active component. The active component of the n-hexane extract was identified to be esters of phthalic acid (esters of 1, 2 benzene dicarboxylic acid) by GC-MS analysis. An authentic sample of n-butyl phthalate was prepared in the laboratory and its characteristics in GC-MS analysis were compared with that obtained for the n-hexane extract of Pithophora oedogonia to confirm the presence of n-butyl phthalate in the extract. The first report on the occurrence and isolation of phthalic acid in plants appears to be from the fungus Gibberella fujikuroi (Cross et al., 1963). Its bioactivity in respect to insect repellent qualities has been studied and its chemical structure is indexed (Pollock et al., 1965; Merck Index, 1996). Phthalic acid esters are also known to be antihelmintic. The risk factors of phthalic acid esters have been assessed and available. However, this compound has not been reported in any marine or fresh water algae. Thus it is the first report on the occurrence and isolation of n-butyl phthalate, an active antifungal compound against phytopathogens from the fresh water alga Pithophora oedogonia. The antifungal nature of the compound is also recorded for the first time.
Acknowledgement
The1st author is grateful to the university grants commission for the award of FDP
References
- Codomier, L., Y. Bruneau., G. Combaut and Teste, J. 1977. Etude biologique et chimique d’ As paragopsis armata et de Falkenbergia runfolanosa. (Rhodophycees, Bonnemai sonniales). C.R. Acad. Sc. Paris. S’erie D. 284 : 1163 – 1165.
- Cross, B.E., John Fredrick grove and Morrison, A. 1963. Gibberellic acid Part XVIII some rearrangements of ring. Chem. Soc. 2 : 2498 – 2521
- Deng – Songzhi and Deng – Sz. 1997. The Research and application of Marine Natural products. Natural product – Research and development.9 (1) : 56 –- 64.
- Fenical, N. 1974. Cycloendesmol, an antibiotic cyclopropane containing sesquiterpene from the marine alga, Chondria opposicloda Dawson J.J.Tetrahedron Letters 13 : 1137– 1140
- Fenical, W.F., Sims, J.J., Squatrito, D., Wing R.N. and Radlick, P. 1973. Izonarol and Isozonarol. Fungitoxic hyroguinones from the brown seaweed Dictyopteris zonarioides. org. chem. 38 : 2383.
- Melo, V.M.M., Medeiros, D.A., Rios, F.J.B., Castelar, L.I.M., Carvelho, A., de Fu 1997. Antifungal properties of proteins (agglutinins) from red alga Hypnea musciformis (wulfen) Lamouroux. Bot. Mar. 40 (4) : 281 – 284.
- Merck index, Bunavari, S. editor 12th ed. White house station, New Jersey: Merck and Co. Inc 1996. pp975 Perry N.B., Blunt J.W., Munro, M.H.G. 1991.
- A cytotoxic and antifungal 1, 4, napthoquinone and related compounds from New Zealand brown algae, Landsubrgia queriafolia. Journal of Natural products 54 (4) : 978 – 985.
- Pollock, J.R.A. and Stevens, R. 1965. Dictionary of organic compounds. Eyre and Spottiswoode Publishers E and f spon Ltd.
- Sharma, G.M., Michaels, L., and Burkholder, P.R., 1968. Goniodomin, a new antibiotic from a dinoflagellate. Antibiot. 21: 659 – 664.

This work is licensed under a Creative Commons Attribution 4.0 International License.





