How to Cite | Publication History | PlumX Article Matrix
R. B. Singh
Department of Zoology, School of Life Sciences, Dr. B.R. Ambedkar University, Khandari Campus, Agra - 282 002 India.
Corresponding Author E-mail: rbsinghugc@gmail.com
ABSTRACT: Upon partial acid hydrolysis of water soluble Rauwolfia serpentina Benth. seeds polysaccharide gave a mixture of three disaccharides and one trisaccharide as : 6-Oa-D-mannopyranosyl-(1®6)-O-a-D-mannopyranose (I); 6-O-a-D-glucopyranosyl-(1®6)-O-a-D-glucopyranose (II); 4-O-b-D-glucopyranosyl-(1®4)-O-b-D-mannopyranose (III) and 4-O-b-D-mannopyranosyl-(1®4)-O-b-D-mannopyanosyl-(1®4)-O-b-mannopyranose (IV).
KEYWORDS: Oligosaccharides; Rauwolfia serpentina Benth; seeds polysaccharide
Download this article as:| Copy the following to cite this article: Singh R. B. Oligosaccharides Structure by Partial Acid Hydrolysis From Rauwolfia Serpentina Benth. Seeds Polysaccharide. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Singh R. B. Oligosaccharides Structure by Partial Acid Hydrolysis From Rauwolfia Serpentina Benth. Seeds Polysaccharide. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8135 |
Introduction
In our earlier communications1 of Rauwolfia serpentina Benth. (Sarpagandha) plant belongs to family- Apoeynaceae is an evergreen undershrub about 15-45 cm in height. The water soluble seeds extract as glucomannan was found to be composed at D-glucose and D-mannose in the molar ratio of 1:2. Nature of the constituent sugars2 methylation periodate oxidation3 and Smith degradation studies of the seeds glucomannan revealed that the D-glucopranose units occupy the terminal position in the main chain which consist of D-mannopyranose unit at the main polymer chain and at the non-reducing end position.The present manuscript mainly deals with the isolation structure elucidation of the oligosaccharides obtained from the Rauwolfia serpentina Benth. seeds glucomannan by partial acid hydrolysis. For identification of oligosaccharides the column chromatography was carried out with partial acid hydrolysis over charcoal-celite column4 and characterisation of sugars by paper chromatographic analysis5 on Whatman No. 3 MM paper afforded three disaccharides and one trisaccharide. These oligosaccharides were characterised by its optical rotation, formation of crystalline derivatives (disaccharides only), degree of polymerization6, reduction with sodium borohydride, complete acid hydrolysis and periodate oxidation studies7. Oligosaccharide were characterised and identified as followes: (I) 6-O-α-D-mannopyranosyl-(1→6)-O-α-D-mannopyranose; (II) 6-O-α-D-glucopyranosyl-(1→6)-O-α-D-glucopyranose; (III) 4-O-β-D-glucopyranosyl-(1→4)-O-β-D-mannopyranose and (IV) 4-O-β-D-mannopyranosyl-(1→4)-O-β-D-mannopyanosyl-(1→4)-O-β-mannopyranose.
Experimental
Separation of oligosaccharides from the hydrolysed compound of Rauwolfia serpentina Benth. water soluble seeds polysaccharide was carried out by descending technique on Whatman No. 3 MM filter paper sheet by paper chromatography8. The upper phase of following solvent mixture (v/v) were used for the detection of monosaccharide and oligosaccharides as : (A) n-butanol-acetic acid-water (4:1:5)9, (B) ethyl acetate-acetic acid-water (9:2:2)10 and (C) ethyl acetate-pyridine water (10:4:3)11. The spray reagent (R) p-anisidine phosphate was used for the detection of monosaccharide and oligosaccharides from the hydrolysed compound of polysaccharide. All evaporation of oligosacchrides was carried out under reduced pressure at 40-500C. The optical rotation values were recoded after equilibrium and melting points are uncontrolled. The Rgal and Rglu refer to the rate of movement of sugars releative to D-galactose and D-glucose respectively. Oligosaccharides mixtures were separated on charcoal-celite column (1:1, w/w) using water followed by 2.5, 5.0, 7.5 and 10.0% (v/v) with aqueous ethanol as eluants. These eluants were further separated by paper chromatography on Whatman No. 3 MM filter paper sheet. The degree of polymerization (DP)13 was determined by Timell’s method and Deionisation was done with Amberlite ion-exchange resins14, IR-45 (OH–) and IR-120 (H+).
Partial acid hydrolysis of oligosaccharides
After a series of trial experiments the following method was carried out for the partial acid hydrolysis15 to obtain the oligosaccharides. Polysaccharide of Rauwolfia serpentina Benth. seeds (18 gm) was hydrolysed with sulphuric acid (1.5 N, 500 ml) for 24 hrs at 4.50C in refrigerator then the content was heated for 50 min. over boiling water bath. The obtained hydrolysate was cooled, filtered and neutralized with barium carbonate slurry. It was again filtered and filtrate concentrated to a small volume about 30 ml. Ethanol about 450 ml was added with the help of mechanical stirrer when the degraded polysaccharides was precipitated out as white coarse power after filtration then dried. The paper chromatographic analysis5 of the hydrolysate syrup on Whatman No. 3 MM filter paper sheet in solvent mixture (A) obtained after concentration of ethanolic extract showed the presence of D-glucose and D-mannose and a member of oligosaccharides.
The degraded polysaccharide was again hydrolysed by keeping it in sulphuric acid (1.5 N, 600 ml) at room temperature for 72 hrs. It was heated on boiling water bath for 1 hr followed by cooling it in the same bath for 30 min then concentrated to a syrup. The syrup was deionised with Amberlite ion exchange resin, IR-45 (OH–) and IR-120 (H–).
Separation of oligosaccharides
The oligosaccharides were seprated16 by chromatographic adsorption method on charcoal-ceilte (1:1, w/w) glass column (60 x 2.5 cm) using the graded elution method4. The Column was eluted with water (2 liters) under 7 lbs/sq. inch pressure to remove the monosaccharides then successively with 3 liters each of 2.5, 5.0, 7.5 and 10.0 % aqueous ethanol (v/v) as eluant. Oligosaccharides fraction (100 ml) was concentrated and then examined by paper chromatographic analysis on Whantman No. 3 MM filter paper sheet in solvent mixture (A) and used (R) as spray reagent. The corresponding sugar strips of oligosaccharides17 were cut out with the help of guide spots and eluted with water according to the Dent’s method then finally concentrated to a thin syrup. It is observed that each fraction was not a single component but a mixture of 4 oligosaccharides. It led to the isolation of oligosaccharides were identified as 3 disaccharides17 and one trisaccharide17 which were characterised as follows:
I: 6-O-α-D-mannopyranosyl-(1→6)-O-α-D-mannopyranose
Sugar syrup (240 mg) had Rgal 0.58 in solvent mixture (B) and Rgla 0.43 in solvent mixture (C) and having optical rotation [α]D24 – 12.4 (H2O) and -20.80C (C2H5OH), Lit [α]D – 12.80C (H2O) and – 210C (C2H5OH) and had m.p. and mixed m.p. 156-1580C19. Degree of polymerization was found to be 2.28 indicating that this oligosaccharide was a disaccharide. Acid hydrolysis of disaccharide with sulphuric acid (1N) showed the presence of D-mannose sugars only which was identified by paper chromatographic analysis. Derivatives of disaccharide (60 mg) was prepared by usual manner as 6-O-α-D-mannopyranosyl –D-mannopyranose-octa-acetate, had m.p. 151-1520C, Lit. m.p. 152-1530C19.
Periodate oxidation of disaccharide (60 mg) was carried out with sodium metperiodate at 4-80C for 55 hrs. It consumed 5.86 moles of periodate oxidant and liberated 3.28 moles of formic acid per mole of disaccharide and results are given in table1.
Table 1: Periodate oxidation of oligosaccharide I.
| S.
No. |
Sugar content | Time (hrs) | ||||||
| 10 | 20 | 30 | 40 | 45 | 50 | 55 | ||
| 1. | Periodate consumption of disaccharide – I (moles/mole) | 2.75 | 3.98 | 5.36 | 5.62 | 5.86 | 5.86 | 5.86 |
| 2. | Formic acid liberation of disaccharide- I (moles/mole) | 1.32 | 2.48 | 2.94 | 3.14 | 3.28 | 3.28 | 3.28 |
6-O-α-D-glucopyranosyl-(1→6)-O-α-D-glucopyranose
Syrup (290 mg) had Rgal 0.66 in solvent (B) and Rglu 0.49 in solvent (C), optical rotation [α]D24+35.00C→ + 20.50C (H2O), Lit [α]D + 34.50C → + 19.90C (H2O)20 and having m.p. 182-1840C. Acid hydrolysis with sulphuric acid (1N) by usual manner to obtained the hydrolysate which was paper chromatographically examined to show the presence of D-glucose only as determined by phenol sulphuric acid method21. Degree of polymerization was formed to be 1.86 indicating that this oligosaccharide was a disaccharide. Derivatives of oligosaccharide was prepared by usual manner as phenyl osazone having m.p. 174-1760C22. Peridate oxidation23 oligosaccharide (50 mg) was carried out with sodium metaperiodate (0.25 M, 10 ml) at 4-80C in refrigerator for 55 hrs. It consumed 5.58 moles of periodate with the simultaneous liberation of 2.82 moles of formic acid per mole of disaccharide after 55 hrs and results are given table.2.
Table 2: Periodate oxidation of oligosaccharide-II.
| S.
No. |
Sugar content | Time (hrs) | ||||||
| 10 | 20 | 30 | 40 | 45 | 50 | 55 | ||
| 1. | Periodate consumption of disaccharide – II (moles/mole) | 2.84 | 3.98 | 5.26 | 5.42 | 5.58 | 5.58 | 5.58 |
| 2. | Formic acid liberation of disaccharide- II (moles/mole) | 1.28 | 1.52 | 1.76 | 2.14 | 2.82 | 2.82 | 2.82 |
4-O-β-D-glucopyranosyl-(1→4)-O-β-D-mannopyranose
Sugar syrup (280 mg) had Rgal 0.64 in solvent (B) and Rglu 0.53 in solvent (C), optical rotation [α]D24+ 15.80C (H2O) and had m.p. 193-1950C. The DP was found to be 1.86 which indicating that the oligosaccharide was a disaccharide. Oligosaccharide was hydrolysed with sulphuric acid (1N) and subsequent paper chromatography of the hydrolyzate indicated that the presence of monosaccharide, D-glucose and D-mannose are in equal amount21. The phenyl hydrazone derivative22 of disaccharide (50 mg) was prepared by usual manner having m.p. 192-1940C19. The periodate oxidation of oligosaccharide (60 mg) by usual manner consumed 5.98 moles of periodate and liberated 3.60 moles of formic acid per mole of disaccharide after 55 hrs and results are shown in table 3.
Table 3: Periodate oxidation of oligosaccharide-III
| S.
No. |
Sugar content | Time (hrs) | ||||||
| 10 | 20 | 30 | 40 | 45 | 50 | 55 | ||
| 1. | Periodate consumption of disaccharide – III (moles/mole) | 2.96 | 4.10 | 5.42 | 5.76 | 5.98 | 5.98 | 5.98 |
| 2. | Formic acid liberation of disaccharide- III (moles/mole) | 1.38 | 2.54 | 3.14 | 3.46 | 3.60 | 3.60 | 3.60 |
4-O-β-D-mannopyranosyl-(1→4)-O-β-D-mannopyranosyl-(1→4)-O-β-D-mannopyranose
Sugar syrup (260 mg) had Rgal 0.32 in solvent (B) and Rglu 0.19 in solvent (C), optical rotation [α]D24+ 210C (H2O), Lit. [α]D – 220C (H2O), having m.p. 167-1690C. Lit. m.p. 169.50C24. Degree of polymerization was found to be 3.14 indicating that the oligosaccharide was a trisaccharide. Methylation studies of trisaccharide by Hakomari’s method on paper chromatography gave presence of 2,3,4,6-tetra-O-methyl-D-mannose and 2,3,6-tri-O-methyl-D-mannose which showed (1→4)-β-type linkages by emulsin. On periodate oxidation studies of the trisaccharide by usual manner consumed 6.18 moles of periodate and liberated 3.56 moles of formic acid per moles of trisaccharide after 55 hrs and results are shown table-4.
Table 4: Periodate oxidation of oligosaccharide-IV
| S.
No. |
Sugar content | Time (hrs) | ||||||
| 10 | 20 | 30 | 40 | 45 | 50 | 55 | ||
| 1. | Periodate consumption of disaccharide – IV (moles/mole) | 3.14 | 4.94 | 5.64 | 5.86 | 6.18 | 6.18 | 6.18 |
| 2. | Formic acid liberation of disaccharide- IV (moles/mole) | 2.26 | 2.82 | 3.24 | 3.42 | 3.56 | 3.56 | 3.56 |
Results and Discussion
Water soluble seeds polysaccharide from Rauwolfia serpentina Benth. (Sarpagandha) obtained as D-glucose and D-mannose in 1:2 molar ratio by paper chromatographic analysis of hydrolysed compound by usual manner. Present manuscript mainly deals with the partial acid hydrolysis of purified seeds polysaccharide with 1N sulphuric acid followed by charcoal-celite column chromatographic analysis and paper chromatography of the hydrolysate afforded three disaccharides and one trisaccharide as major components. Oligosaccharides were purified separately and characterised by their optical rotation melting points, formation of crystalline derivatives (disaccharides only), degree of polymerization (DP), complete acid hydrolysis and periodate oxidation studies. The oligosaccharides were identified as : (I) 6-O-α-D-mannopyranosyl-(1→6)-O-α-D-mannopyranose; (II) 6-O-α-D-glucopyranosyl-(1→6)-O-α-D-glucopyranose; (III) 4-O-β-D-glucopyranosyl-(1→4)-O-β-D-mannopyranose and (IV) 4-O-β-D-mannopyranosyl-(1→4)-O-β-D-mannopyanosyl-(1→4)-O-β-mannopyranose. Structure of oligosaccharides from Rauwolifa serpentina Benth. seeds polysaccharide are shown in Figure-1.
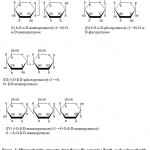 |
Figure 1: Oligosaccharides strucutre from Rauwolfia serpentina Benth. seeds polysaccharide.
|
Isolation of oligosaccharide (III) and (IV) clearly indicates that the main chain or backbone of the glucomannan polymer contains D-glucopyrannose and D-mannopyranose units which are joined through (1→4)-β-type linkages. Formation of oligosaccharide (I) and (II) suggests that the branches at main chain consists of no-reducing end with D-mannopyranose and D-glucopyranose residues which are glycosidically attached through (1→6)-α-type linkages with mannospyranose & D-mannopyranose and D-glucopyranose & D-glucopyranose. Further the trisaccharide (IV) was also confirms that the D-mannopyranose units at the main chain by (1→4)-β-type linkages. The earlier proposed polysaccharide structure of Rauwolfia serpentina Benth. seeds polysaccharide (Figure-2) obtained after methylation results is favoured by the above oligosaccharide results.
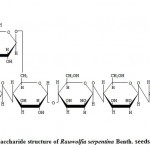 |
Figure 2: Polysaccharide structure of Rauwolfia serpentina Benth. seeds polysaccharide.
|
References
- Krishnamurthi, A. Wealth of India Raw Materials. Publication & Information Directorate, CSIR, New Delhi (India), 8 (Ph-Re): 376 (1969).
- Pathak, D.K.; Goyal, M.M. and Singh, R.B. Extraction and identification of water soluble polysaccharide in Rauwalfia serpentina seeds. J. Chemtracks, 9 (1 & 2): 129 (2007).
- Singh R.B. Periodate oxidation studies from medicinal plant of Rauwolfia serpentina seeds polysaccharide, J. Chemtracks, 9 (1&2): 241 (2007).
- Kowkobany, G.N. Advance in Carbohydrate Chemistry, 9: 304 (1950).
- Binkley, W.W. Advance in carbohydrate chemistry, 10: 55 (1955).
- Timell, T.E. and Svensk, V. Stid., 63 : 688(1960).
- Fluery, P. and Lange, J. Identification of oligosaccharides by periodate oxidation method. Pharm. Chem., 17 : 107 (1933).
- Partridge, S.M. Nature (London), 158 : 270 (1946).
- Partridge, S.M. and Westall, R.G. J., 42: 238 (1948).
- Jermyn, M.A. and Isherwood, F.A. J., 44: 402 (1949).
- Meier, H. Identification of polysaccharide by desensing technique on paper chromatography, Acta Chem-Scand. 14: 749(1960).
- Mukherjee, S. and Srivastava, H.C. Identification of Guar gum galactomannan by paper chromatography. Nature (London), 169: 330 (1952).
- Timell, T.E. and Svensk, V. Stid., 63: 688 (1960).
- Adams, B.A. and Halmes, E.L. Ion exchange resins of oligosaccharide, Soc. Chem. Ind., 54: 17 (1935).
- Parkin, V.M. and Jones, J.K.N. Partial acid hydrolysis of oligosaccharides. J. Chemistry, 44: 1531 (1966).
- Whistler, R.L. and Durso, D.F. Amer. Chem. Soc., 73: 4189 (1951).
- Andrews, P., Hough, L. and Jones, J.K.N. Mannose containing polysaccharide-I. The galactomannan of Lucerne and Clover J. Amer. Chem. Soc., 74: 4029 (1952).
- Dent, C.E. Identification of sugars by paper chromatography, Biochem, J., 41: 240 (1947).
- Wolform, M.L. and Word, H.B. Amer. Chem. Soc., 73: 2933 (1951).
- Hassid, W.I. and Ballou, C.E. Cabohydrate (oligosaccharides), Academic Press, New York, 9: 511 (1957).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A. and Smith, F. Colorimetric method for the determination of sugars and related substances, Chem., 28: 350 (1956).
- Rafique, C.M. and Smith, F. The constitution of Guar J. Amer. Chem. Soc., 72: 4034 (1950).
- Abdel, A.M. and Smith, F. Amer. Chem. Soc., 73: 994 (1952).
- Tyminski, A. and Timell, T.E., Amer. Chem. Soc., 82: 2823 (1960).

This work is licensed under a Creative Commons Attribution 4.0 International License.





