How to Cite | Publication History | PlumX Article Matrix
Possible control of fungal and insect infestation of date fruits using ozone
Saeed S. Al-Ahmadi, Reda A. Ibrahim and Salama A. Ouf
Department of Biology, Faculty of Science, Taibah University, Madinah Mounawara, P.O. 30002 Saudi Arabia.
ABSTRACT: Fruits of ten date palm cultivars, collected from the area of Almadinah Almunawwarah, Saudi Arabia were tested for their fungal and insect infestation. Fifteeen fungal species and three insect pests were recorded from the tested cultivars. The fungal infestation of date cultivars can be arranged as follow: Baeiddy > Anbrah > Rothan > Berni > Ajwah > Shalaby > Safawy > Labban > Rabbiah > Sokai. Aspergillus, paecilomyces, Penicillium and Fusarium were the dominant genera. The recovered insects were Oryzaephilus surinamensis, Oryzaephilus Mercator, Cadra furcatella baptella. The highest insect damages were recorded in the case of Safway cultivar infested with C. furcatella baptella (8.16%) followed by Sokai and Barni cultivars infested with O. surinamensis (7.83 and 6.33%, respectively). The growth of most test fungi was significantly decrease on exposure to 4 ppm ozone for 120 minutes and steady drop in growth rate was achieved on dose elvevation to 8 ppm accompanied with extending the exposure time. Ozone concentration of 8 ppm was lethal for all fungal species, when the exposure time extended to 180 or 240 minutes. There was a steady increase in mortality of larvae and adults of O. surinamensis reaching 100% at 30 ppm ozone delivered for 6 hours. Eggs and pupae were relatively sensitive to ozone as compared to larvae and adults where 100% mortality was achieved using 7 ppm ozone for one hour.
KEYWORDS: Ozone; date fruits; infestation, fungi; insects; Oryzaephilus surinamensis
Download this article as:| Copy the following to cite this article: Al-Ahmadi, S. S, Ibrahim R. A, Ouf S. A. Possible control of fungal and insect infestation of date fruits using ozone. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Al-Ahmadi, S. S, Ibrahim R. A, Ouf S. A. Possible control of fungal and insect infestation of date fruits using ozone. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=7803 |
Introduction
Date palm (Phoenix dactylifera) is an important traditional crop in the kingdom of Saudi Arabia. The number of trees in the kingdom is estimated to be over 13 million with an annual production of approximately 563 000 tons of dates (Ministry of Agriculture, 1996). The dates are marketed allover the world through the pilgrims and visitors. Dates are produced in comparatively short periods with the tendency of production peaks during the harvest season in summer. In order to store the date for a long period (several months to one year), it must be packed after completely cleaned from any contaminants. It has been reported that several fungi and insect pests cause the rot and/or damage of date during inadequate processing, packing and storage. Al-Ahmadi (1986) reported that the dry dates in Madinah region are infested by certain insect pests belong to orders Coleoptera (O. surinamensis and Oryzaephilus mercator), and Lepidoptera (Cardra furctella).
The rotting depends on the date quality, palm species, cellulolytic activity of the contaminant, and prevailing environmental conditions. The insects play an important role in dispersion of the contaminated microbes during fruit storage and rendering the date non-edible. Moreover, some of contaminating fungi may be regarded as potentially dangerous pathogens of date-palm due to production of toxic substances (mycotoxins) that diffuse in flesh of fruit causing severe problems to human health.
The packing of date involves fumigation with methyl bromide, fungicides or pesticides. However, the sanitizers are dangerous poisons and has been limited or banned by most of international regulations concerning the potential health hazards and depletion of ozone layer (Council on Radiation Application, 1985).
The need for potent and safe antimicrobial and pesticidal agents has increased in recent years due to increasing disease outbreaks, emergence of new foodborne pathogens and illnesses arising from the presence of some microbes in frozen food. Ozone is known to act as strong antimicrobial agent against bacteria, fungi and viruses. It is a powerful oxidant that has numerous beneficial applications. Ozone has been used to sterilize a range of substances including air (Xu et al., 2002), waste water, swimming pool water, drinking water (Greene et al., 1993) and against microflora on meat, poultry, eggs, fish, fruits and vegetables (kim et al. 1999). However, the use of ozone as a decontamination gas for dried fruits has not been well studied.
The objectives of this study were to determine the infestation of different date fruit cultivars with fungi and insect pests and the efficacy of ozone in inhibiting growth of the isolated fungi and in inducing mortality of the detected pest insects in an attempt to use ozone in disinfecting the dates from the associated fungi and insects.
Materials and methods
Test samples
Fruits of ten cultivars of dates namely, Barni Mabroom, Baeiddy, Shalaby, Safawy, Sokai, Ajwah, Anbrah, Rabbiah and Rothana were used in this study. The samples were collected, in sterile plastic bags, from different localities in Madinah , Saudi Arabia, and stored in refrigerator until use.
Fungal isolation
Isolation of fungi was carried out from the naturally contaminated datese. Czapek–Dox’s agar media (Dox, 1910) was used as isolation medium. It has the following composition (g/L): 20 sucrose; 20 agar; The dilution plate method as described by Johnson et al., (1960) was adopted for counting of fungi. Pieces of tissues from each sample (1 cm X 1 cm) were dipped momentarily into a 0.5 % (m/v) calcium hypochlorite solution and four pieces (about 10 g) were mixed with 90 ml of sterile distilled water and shaked vigorously. Suitable dilutions were made for each plant sample. After solubilization and sterilization of the medium, streptomycin 30 mg/ml was added. Fifteen ml of this medium were cooled to just above the solidification and added to each Petri-dish. One ml from the prepared dilution of each plant sample was transferred aseptically into each of six petri-dishes containing isolation medium. The dishes were rotated by hand in a broad swirling motion so that the diluted samples were dispersed in agar. After incubation at 28oC for 7 to 15 days, the resulting colonies were estimated per gram dry material. The developing fungal colonies were identified up to the species level by microscopic examination. This was made through the help of the references of Barnett (1960), Barron (1968), Ellis (1971,1976), Kendrick (1971), Moubasher (1993), Raper and Fenell (1965), Samson (1979), (Pitt, 1979), Klick and Pitt (1982) and Robert et al. (1996).
Laboratory insect rearing
The insects infesting date fruits were recovered under the insectary conditions (25±2ºC, 75±5% R.H. and 16h of illumination per day) from naturally infested dates. The fruits were placed in plastic pots (15 cm diameter and 20 cm deep). The pots were then covered with muslin or cheese-cloth fastened by a rubber-band to prevent the escape of insects and to ensure the proper ventilation. The emerged insect species obtained from the culture were counted and the percent infestation rate calculated as the number of infested date fruits manifesting typical insect damage, 45 days after incubation per 100 fruits / replicate was estimated.
The preliminary results revealed that Oryzaephilus surinamensis (Coleoptera: Silvanidae) was the most dominant insect recovered from date fruits. For mass rearing, O. surinamensis was reared on date fruits under the insectary conditions described before. Newly emerged adults, as well as eggs, pupae and larvae obtained from the culture were used to test their susceptibility to ozone.
Ozone treatment and growth criteria of some isolated fungi
Ozone production
Ozone was generated via a controlled flow of oxygen through a corona discharge in the ozone generator (Ozomaxe, Egypt, ozo- 3vtt). The ozone was fed into both chambers where the ozone measurement and ozone treatment were done. Ozone measurement was done by an ozone analyzer (Inusa, H1, ver 5.73) with a detection limit of 1.0 ppb. Preliminary test showed that ozone concentration less than 4 ppm was less effective at different exposure times, so ozone concentrations of 0 (control), 4 and 8 ppm were employed for testing the growth rate and 6 ppm was used for testing sporulation. The exposure time of the inoculum was 0, 60, 120, 180, and 240 min.
Fungal growth rate
Known volumes of Dox medium were sterilized by autoclaving. Aliquots of about 15 ml of this medium were dispersed into sterile petri-dishes, (9 cm diameter). Each dish was inoculated at its center with ozone treated fungal disc (10 mm diameter). The test fungi were Alternaria humicola, Aspergillus flavus, Fusarium moniliforme, Penicillium chrysogenum, Rhizopus oryzae, and Trichoderma viride. Five plates for each treatment were used. The plates were incubated for 10 days during which the colony diameters (in mm) were daily measured (mean of two diameters at right angles to each other). The rate of growth was calculated for each treatment.
Ozone treatment of the test insect
Larvae and adult treatment
The experiment was conducted in two separate lines in the same time for larvae and adults of Oryzaephilus surinamensis. Each treatment consisted of 50 individuals divided into 5 replicates; each comprised 10 either larvae or adults in a transparent plastic container (6.5 cm diameter) covered with muslin. The ozone concentrations used were 5, 10, 20, 30, 40, 60, 80, 100 and 120 ppm for 1 hour exposure time against larvae and adults. The second treatment was carried out by using only one ozone concentration (30 ppm) and different exposure times i.e. 2, 4 and 6 hours. For ozonation, transparent plastic containers of each treatment were placed in a well closed glass box. As control ten larvae and ten adults were placed in two transparent plastic containers under the same conditions but without ozone. The feeding of both larvae and adults of O. surinamensis took place via adding 3 discs of dates (1.2 cm diameter) in each container. Two days after treatment, treated insects were daily investigated and dead ones were recorded and segregated. Mortality percentage was determined.
Eggs and pupae treatment
As a result of the preliminary bioassay experiments which showed that, both eggs and pupae of O. surinamensis were more sensitive to the toxic effect of ozone. Therefore, the ozone concentrations used against eggs and pupae were 1, 3, 5 and 7 ppm for 1 hour exposure time. Three treatments each consists of 50 eggs or pupae. Each treatment divided into 5 Petri dishes (replicates) each 10 eggs or pupae. For ozonation, Petri dishes of each treatment were placed in a well closed glass box. Two days after treatment, both treated stages were daily investigated and dead one and/or malformed were recorded and segregated. Ten eggs and ten pupae were used as control. Mortality percentage was determined. Statistical analysis was performed by using ANOVA multiple mean comparisons were made by the Tukey-HSD-Test.
Results
Isolation of fungi and insect recovery
Isolation of fungi
Table 1 indicates that the fruits of Baeiddy and Anbarah were the highest in fungal infestation (126.0 and 112.2 colonies/gram) while Sokai and Rabbiah were the lowest (22.8 and 48.3 colonies/ gram). The genus Aspergillus represented the fungus of the highest population density and occurrence being isolated from all test date fruits (Table 2). The total count of Aspergillus was 393.7 colonies/g representing 50.54% of the total fungal population. The highest counts were recorded from Rothan (55.4 colonies/g) followed by Rabbiah (51.5 colonies/g) and Berni (49.0 colonies/g) . The genus Aspergillus was represented by 4 species of which A. niger was the highest in population density and counted 208.0
colonies/g, constituting 26.70 of the total fungal population. A. niger was of high occurrence being isolated from all of the ten test plant materials.
flavus and A. fumigates were lower in population density than A. niger and counted 82.7 and 82.2 colonies/g constituting 10.62% and 10.68% of the total population. Both Aspergilli were of high occurrence. A. ustus was of low occurrence being recovered Berni, Shalaby, Safawy, Anbarah and Rothan.
The genus Paecilomyces, represented by only one species namely P. divericata, ranked second in the order of population density and constituted 94.5 colonies/g (12.13% of total population). The species was of moderate occurrence being recovered from 7 samples.
The genus Penicillium ranked third in the order of population density. The count of Penicillium spp. was 92.0 colonies/g which represented 11.81% of the total fungal count. Penicillium was represented by two species in which P. citinum (62.8 colonies constituting 8.06%) dominated P. chrysogenum (29.2 colonies constituting 3.75%). P. citrinum was recovered from 7 cultivars, while P. chrysogenum was isolated from 4 cultivars.
The genus Fusarium ranked fourth according to its total count (54.7 colonies/g) constituting 7.03% of the total population. The genus was represented by 2 species, F. moniliforme and F. oxysporum with counts of 35.1 and 19.6 colonies/g representing 4.51% and 2.52% of the total fungal population, respectively. Fusarium species were isolated from 7 cultivars, five for the former and two for the later species.
The genus Mycospaerella hold fifth in the order of population density. The count of Mycospaerella was 39.7 colonies/g which represented 5.10% of the total fungal count. M. tassiana, the only isolated species, was recorded in moderate occurrence from five cultivar fruits.
Alternaria came next to Mycospaerella in order of population density where its count was 26.2 colonies/g constituting 3.36% of the total fungal count. The genus was represented by A. alternate recovered in low occurrence being isolated from 4 cultivar fruits
The genus Rhizopus, represented by R. oryzae, ranked seventh in the order of total count. Its count was 21.9 colonies/g plant material which constituted 2.81% of the total fungal population. R. oryzae was isolated in moderate occurrence from five cultivar fruits.
Ulocladium atrum and Mucor racemosus came next according the order of total population, where their counts were 18.9 and 13.0 colonies/g constituting 2.43and 1.67%, respectively. Each of the two species was recovered from four samples.
Table 1: Count of fungal species (colony /gram fresh material) isolated from different cultivars of date fruits using Czapek` s Dox medium.
| Fungal species | Berni | Cultivar | |||||||||
| Alternaria alternata | 11.8 | Baeiddy | Shalaby | Safawy | Sokai | Ajwah | Anbrah | Rabbiah | Rothan | Labban | Total count |
| Aspergillus flavus | 8 | 0 | 0 | 0 | 0 | 1.2 | 10 | 0 | 0 | 3.2 | 26.2 |
| Aspergillus fumigatus | 12 | 12.2 | 11 | 8.8 | 1.5 | 8.2 | 7.8 | 8.8 | 12.2 | 4.2 | 82.7 |
| Aspergillus niger | 25 | 10 | 10.8 | 15 | 2 | 12 | 13.2 | 0 | 8.2 | 0 | 83.2 |
| Aspergillus ustus | 4 | 32 | 18.8 | 13.3 | 11.5 | 16 | 22 | 20.6 | 32 | 16.8 | 208 |
| Cladosporuim herbarum | 0 | 0 | 2.5 | 1.8 | 0 | 0 | 8.5 | 0 | 3 | 0 | 19.8 |
| Fusarium moniliforme | 14 | 12 | 0 | 6.3 | 0 | 0 | 2.2 | 3.9 | 0 | 0 | 24.4 |
| Fusarium oxsporum | 0 | 8.4 | 0 | 0 | 1.8 | 6.4 | 0 | 4.5 | 0 | 0 | 35.1 |
| Paecilomyces divaricata | 12 | 0 | 11.4 | 0 | 0 | 0 | 0 | 0 | 0 | 8.2 | 19.6 |
| Penicillium chrysogenum | 0 | 18.9 | 0 | 9 | 0 | 12.8 | 17.2 | 0 | 15.6 | 9 | 94.5 |
| Penicillium citrinum | 8.5 | 0 | 6.4 | 0 | 0 | 6.6 | 9.8 | 0 | 6.4 | 0 | 29.2 |
| Rhizopus oryzae | 2 | 11.5 | 0 | 0 | 0 | 9.8 | 12.8 | 6.5 | 8.1 | 5.6 | 62.8 |
| Mucor racemosus | 0 | 7.5 | 3 | 0 | 3.6 | 0 | 0 | 0 | 5.8 | 0 | 21.9 |
| Mycospaerella tassiana | 0 | 0 | 5 | 2.4 | 2.4 | 0 | 0 | 0 | 0 | 3.2 | 13 |
| Ulocladium atrum | 2.5 | 13.5 | 6.3 | 0 | 0 | 4.4 | 8.7 | 0 | 6.8 | 0 | 39.7 |
| Total count | 99.8 | 0 | 0 | 0 | 0 | 6.2 | 0 | 4 | 6.2 | 0 | 18.9 |
| 126 | 75.2 | 56.6 | 22.8 | 83.6 | 112.2 | 48.3 | 104.3 | 50.2 | 779 |
Table 2. Percent of total population and frequency of occurrence of the fungal species isolated from different cultivars of date fruits.
|
Fungal species
|
% of total population |
Cases of isolation |
Occurrence |
Alternaria alternata |
3.36 | 4 | L |
Aspergillus flavus |
10.62 | 10 | H |
| Aspergillus fumigatus | 10.68 | 10 | H |
| Aspergillus niger | 26.70 | 10 | H |
| Aspergillus ustus | 2.54 | 4 | L |
| Cladosporuim herbarum | 3.13 | 4 | L |
| Fusarium moniliforme | 4.51 | 5 | M |
| Fusarium oxsporum | 2.52 | 2 | R |
| Paecilomyces divaricata | 12.13 | 7 | M |
Penicillium chrysogenum |
3.75 | 4 | L |
| Penicillium citrinum | 8.06 | 7 | M |
| Rhizopus oryzae | 2.81 | 5 | M |
| Mucor racemosus | 1.67 | 4 | L |
| Mycospaerella tassiana | 5.10 | 5 | M |
| Ulocladium atrum | 2.43 | 4 | L |
Frequency of occurrence according to cases of isolation:
8-10 cases = High occurrence (H)
5-7 cases = Moderate occurrence (M)
3-4 cases = Low occurrence (L)
1-2 cases = Rare occurrence (R)
Insect rearing
Three insects, at least in one of their developmental stage, were recorded infesting the different date fruit cultivars, namely Oryzaephilus surinamensis, Oryzaephilus Mercator and Cadra furcatella baptella (Table 3). The different cultivars can be arranged in descending order according to the infestation rate with O. surinamensis as follow: Sokai > Barni > Baeiddy > Anbarah > Rothan > Safawy and Lebban > Shalaby > Ajwah and Rabbiah. In case of O. Mercator, the infestation rate was Safawy > Barni > Rabbiah > Ajwah > Baeiddy > Shalaby > Anbarah > Labban > Sokai > Rothan, and it was Safawy > Anbarah and Labban > Shalaby > Ajwah > Baeiddy and Sokai > Rothan > Rabbiah > Barni in the case of C. furcatella baptella .
Table 3. Percent infestation rate calculated as the number of contaminated
date fruits manifesting typical insect damage, 45 days after
incubation at 25ºC ± 2 ºC per 100 fruits / replicate.
| Causal insect | |||
| Cultivar | |||
| Oryzaephilus surinamensis | Oryzaephilus mercator | Cadra furcatella baptella | |
| Barni | 6.33±2.4 | 3.63±2.3 | 2.13±1.3 |
| Baeiddy | 5.66±2.1 | 2.46±1.3 | 3.16±1.9 |
| Shalaby | 2.17±1.7 | 2.24±0.8 | 4.02±1.2 |
| Safawy | 3.16±2.7 | 4.36±2.5 | 8.16±1.6 |
| Sokai | 7.83±1.2 | 1.71±1.1 | 3.16±1.2 |
| Ajwah | 2.16±1.1 | 2.56±1.5 | 3.66±1.1 |
| Anbrah | 5.08±.01 | 2.23±0.9 | 4.33±1.4 |
| Rabbiah | 2.16±0.2 | 2.86±1.1 | 2.83±1.2 |
| Rothan | 4.16±0.3 | 1.26±0.4 | 3.01±0.9 |
| Labban | 3.16±1.2 | 1.43±0.6 | 4.33±1.2 |
The highest infestation rate was recorded in the case of Safway cultivar infested with C. furcatella baptella (8.16%) followed by Sokai and Barni cultivars infested with O. surinamensis (7.83 and 6.33%, respectively). On the other hand, the lowest infestation rate was estimated in the case of Rothan, Labban and Sokai infested by O. Mercator (1.26, 143 and 1,71%, respectively).
Fungal growth rate
At 4 ppm ozone concentration, there was a significant decrease in the growth rate of all test fungi, except Aspegillus flavus, when exposed to 120 minutes. All test fungi were more susceptible and their growth was significantly reduced on exposure to 240 minutes (Fig 1). Ozone concentration of 8 ppm was lethal for all fungal species, when the exposure time extended to 240 minutes although F. oxysporum failed to grow when exposed to 120 min at that concentration.
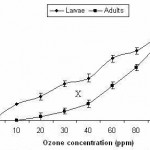 |
Figure 1
|
Mortality of different stages of Oryzaephilus surinamensis
Fig 2 shows that ozone concentration of 5 ppm delivered for one hour does not cause any harmful effect for larvae and adults of Oryzaephilus surinamensis. At 10 ppm, adults were still resistant although larval mortality reached 18.1%. With elevation of ozone concentration, there was a gradual increase in % mortality reaching, at 100 ppm, 82.8 and 86.6% for larvae and adults, respectively. The complete lethal effect of ozone for larvae and adults was induced at 120 ppm.
 |
Figure 2
|
To investigate the effect of prolonged exposure of ozone on mortality of larvae and adults, 30 ppm ozone was delivered for 2, 4 and 6 hours (Fig. 3). The data show that there was a steady increase in mortality of larvae and adults with the extension of exposure time to ozone reaching 100% after 6 hours.
 |
Figure 3
|
Eggs and pupae were susceptible to ozone as compared to larvae and adults (Fig. 4). Hundred percent mortality for eggs and pupae was achieved using 7 ppm ozone for one hour.
 |
Figure 4
|
Discussion
All test dates are contaminated with varying loads of fungi. Baeiddy and Anbarah cultivars were the highest in fungal infestation (126.0 and 112.2 colonies/gram) while Sokai and Rabbiah were the lowest (22.8 and 48.3 colonies/ gram). It is believed that the bulk of the contaminated microbes are probably associated with the investigated plants before harvesting and are varied according to the variation of climatic factors and the methods of handling, transport and storage. (Frazier and Westhoff, 1988).
The genus Aspergillus represented the fungus of the highest population density and occurrence being isolated from all test date fruits. The total count of the represented 50.54% of the total fungal population. The highest counts were recorded from Rothan (55.4 colonies/g) followed by Rabbiah (51.5 colonies/g) and Berni (49.0 colonies/g) . Aspergillus was followed by Paecilomyces (12.13%), Penicillium (11.81%) and Fusarium (7.03%). The other isolated genera namely, Alternaria, Rhizopus, Ulocladium and Mucor, each was isolaled in less than 5% of total population. Concerning species level, A. niger was the dominant (26.70%), followed by P. divericata, A. fumigates (10.68), A. flavus (10.62%), and P. citrinum (8.06%). Several authors reported Aspergillus species as universal inhabitants of dried fruits (Abarca et al. 2003, Magnoli et al. 2003, Romero et al. 2005). Abu Zinada and Ali (1977) in KSA reported that, Aspergillus niger, A. flavus, Rhizopus stolonifer, Penicillium spp., Fusarium sp and Stemphylium verruculosum were the most common fungi associated with dates. Amalaradjou and Venkitanarayanan (2008) detected Penicillium, Aspergillus and Alternaria species in fruits and vegetables. From date fruits in Iran. Elarosi et al. (1983) reported that, fungi belonging to the genera Alternaria, Aspergillus, Aureobasidium, Botryodiploida,Cladosporium, Fusarium, Nigrospora, Paecilomyces and Penicilliumwere frequently isolated from date fruits showing signs of preharvest infections. Nassar (1986) isolated Aspergillus represented by three species, A. niger and 2 species from Aspergillus glaucus group namely A. ruber (= Eurotium rubrum) and A. amstelodami (= E. amstelodami) from dates in Aswan, Egypt.
Concerning the susceptibility of date fruits of different cultivars to the natural insect infestation, the data revealed that all test cultivars were infested at different degree with Oryzaephilus surinamensis, O. mercator and Cadra furcatella baptella. Sokai and Barni were the most susceptible cultivars to O. surinamensis, safway and Barni to O. mercator and Safway, Anbrah, Labban and Shalaby to C. furcatella baptella. The variation in quantitative infestation of cultivars to natural infestation may be due to the variation in chemical composition of fruits. Clifford et al. (1998) reported that accumulation of total amino acids was acceptable for feeding some insect larvae. This accumulation of amino acids may play a role in increasing infestation. Al-Dosari et al. (2002) found that the relationship between infestation by O. surinamensis and protein content was significantly positive. However, Ali and Aldosari (2007) indicated that differences between the date mite infestation and date fruit contents of different cultivars (lipids, proteins, carbohydrates and ash) were insignificant.
In a trail to evaluate the efficacy of gaseous ozone as a sanitizing agent for the investigated plant materials, the research was then directed to in vitro demonstration of the effect of 4 and 8 ppm ozone applied for different exposure times (0-240 minutes) on growth rate of 6 selected fungal species recovered from test dates. The mycelial growth rate of all test fungi affected variably with application of ozone. The magnitude of reduction in growth rate appears to depend on the fungal species, ozone concentration and exposure time. Fusarium oxysporum, was the most susceptible to ozone. All fungi failed to grow at 8 ppm ozone applied for 240 minutes. The differential sensitivity of the test fungi to ozone may primarily be related to different mycelial resistance to ozone penetration. It is believed that ozone, being a potent oxidant, may inactivate the test fungi by alteration in cell wall and/or protoplasmic components. According to Komanapalli and Lau (1996) viability of E. coli decreased with a progressive degradation of intracellular proteins on long exposures to 600 ppm ozone up to 30 minutes. Ozone may also inactivate microorganisms by causing damage to their genetic material. In studies by Prat et al. (1968) and Scott (1975) on DNA of E. coli, the pyrimidine bases were modified by ozonation, with thymine being more sensitive to ozone than cytosine and uracil.
The reactivity of ozone is assumed to be due to the oxidizing power of free radicals formed in a chain reaction during its decomposition. Since organic matter may inhibit this chain reaction ( Hoigne and Bader, 1976), therefore, the differential activity of ozone against the test fungi might be due to the variation in their organic matter content which may accelerate or reduce the toxicity of ozone. This suggestion is recommended by Morin et al., (1993) which found that the specific interaction of sucrose or exopolysaccharides with ozone affects the ozone activity.
The inhibition of mycelial growth and sporulation of Penicillium on citrus fruit due to the oxidizing action of ozone was reported many years ago (Harding, 1968). Later, Krause and Weidensaul (1978) found that relatively low ozone concentration (0.30 µL/L) reduced the virulence of Botrytis cinerea conidia. More recently, Liew and Prange (1994) found that ozone-enriched atmosphere delayed mycelial growth of B. cinerea and Sclerotinia sclerotiorum on carrots. Similar effect on Rhizopus stolonifer was observed on grapes (Sarig et al., 1996). Margosan and Smilanick (1998) reported that germination of B. cinerea, Monilinia fruticola, and Penicillium digitatum spores was inhibited by exposing them to a high ozone concentration (1.30 µL/L) for 80 minutes.
The antimicrobial activity of ozone has long been known. Less clear is its mode of action. Suggestions for primary targets include unsaturated lipids in the cell surface, enzyme sulfhydrayl groups, nucleic acid, and others. Earlier in 1954, Giuese and Christenser, working with bacteria, suggested that the bacterial cell surface is the primary target of ozone activity.
The spore production of all test fungi was reduced on exposure to 6 ppm ozone and the reduction was more pronounced on extension of exposure time. The maximum reduction in spore production was achieved after 240 minutes exposure and ranged from 77.88% in the case of in case of A. alternata to 81.56% in the case of A. flavus. The mycelium of A. niger and F. oxysporum failed to form spores on exposure for 240 minutes. The efficacy of ozone to suppress fungal sporulation is well documentated in reports of Palou et al. (2003), Mason et al (1997), Krause and Weidensaul (1978) and Harding (1968). Heagle and Strickland (1972) observed distoration and plasmolysis of conidia when exposed to 0.2 ppm ozone and suggested that ozone might entered directly into the conidia or conidiophore.
The larvae and adults of Oryzaephilus surinamensis are more susceptibile to long exposure of lower doses than short exposure of higher dose. For example, 100% mortality can be either induced after one hour exposure at 120 ppm or after 6 hours exposure at 30 ppm. On the other hand eggs and pupae were sensitive to ozone as compared to larvae and adults and 100% mortality was achieved using 7 ppm ozone for one hour. Moreover, ozone treatment displayed altered behaviors such as more than one pair of legs failing to move or a lack of coordinated movement in all legs. Erdman (1980) observed mortality of larvae of red flour beetle,Tribolium castaneum (Herbst), and confused flour beetle, Tribolium confusum (du Val), when exposed to a 45 ppm ozone environment. In a laboratory study, 5 ppm of ozone resulted in 100%
mortality of adult saw-toothed grain beetle, Oryzaephilus surinamensis (L.), and confused flour beetle after exposure times of 3 and 5 days, respectively (Mason et al., 1997). Kells et al. (2001) reported that treatment of 8.9 tonnes (350 bu) of maize with 50 ppm ozone for 3 days resulted in 92–100% mortality of adult red flour beetle, Tribolium castaneum (Herbst), adult maize weevil, Sitophilus zeamais (Motsch.), and larval Indian meal moth, Plodia interpunctella (Huebner).
References
- Abarca, M.L., Accensi, F., Bragulat, M.R., Castella´, G., Caban˜ es, F.J. (2003). Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. Journal of Food Protection 66: 504–506
- Abu Zinada, A.H. and Ali, M.I. (1977). Fungi associated with dates in Saudi Arabia. Second International Mycological Congress 27 August- 3 Sept., 1977. University of South Florida Tampa, Florida, U.S.A.
- Al-Ahmadi, S. S. (1986). Studies on some pests of date of Madina Munawwara region. M.Sc. Thesis. Department of Natural Science, Faculty of Education, Madina Munawwara, King Addulaziz University.
- Al-Dosari, S. A., Al-Suhaibani, A.M. and Ali, A.G. (2002). Susceptibility of some dry palm varieties to infestation by Oryzaephilus surinamensis L. (Coleoptera: Silvanidae) in relation to chemical composition. Assiut J. Agri. Sci 33: 1-9
- Ali, A. G. and Al-Dosari, S.A. (2007). Susceptibility of date palm fruit cultivars to the natural infestation by Oligonychus afrasiaticus (Mcg.) (Acari: Tetranychidae) in relation to their chemical composition. Ass. Univ. Bull. Environ.Res. 10: 1-5
- Amalaradjou, M.A.R. and Venkitanarayanan, K. (2008). Detection of Penicillium, Aspergillus and Alternaria species in fruits and vegetables. Mycotoxins in Fruits and Vegetables (2008): 225-247.
- Bancroft, W.D. and Richter, G.H. (1931). The chemistry od disinfection. Journal of Physical Chemistry 35: 511-530.
- Barnett, H. L. (1960). Illustrated genera of imperfect fungi. Burgess publishing company, Minneapolis, pp. 225.
- Barron, G. L. (1968). The genera of Hyphomycetes from soil. Williams and Wilkins, Baltimore.
- Clifford, S. Arndt, S. K., Corlet, J.E., Narhila, N.S., Popp, M. and Jens, H.G. (1998). The role of solute accumulation, osmotic adjustment changing cell wall elasticity in drought tolerance Zizphus maurtiana (Lomk). J. Exp. Bot. 49: 967-977.
- Council on Radiation Application (1985). Atomic industrial Forum, Bethesda, MD.
- Dox, A. W. (1910). The intracellular enzymes of Penicillium and Aspergillus species, special refrence to those of P. camenberiti U.S. Department of Agriculture and Animal Bulletin, 120, 170.
- Elarosi, H., Mussa, A.E.A. and Jaheen, N. (1983). Date fruit rots in the eastern province of Saudi Arabia. Proceeding of the First Symposium on the date palm in Saudi Arabia.
- Ellis, M. B. (1971). More Dematiaceous Hyphomycetes. Commonwealth, Mycol. Inst,, Kew.
- Ellis, M. B. (1976). Dematiaceous Hyphomycetes. Commonwealth, Mycol. Inst,, Kew.
- Erdman, H.E., 1980. Ozone toxicity during ontogeny of two species of flour beetles, Tribolium confusum and T. castaneum. Environmental Entomology 9, 16–17.
- Frazier, W.C. and Westhoff, D.C. (1988). Food Microbiology. 4th edition. McGraw-Hill Publication Company, NewYork, USA.
- Giuese, A. C. and Christenser, E. (1954). Effects of ozone on organisms. Physiology. Zool., 27: 101-115.
- Greene, A. K., Few, B. K. and Serafini, J. C. (1993). A comparison of ozonation and chlorination for the disinfection of stainless steel surfaces. Jouranl of Dairy Science, 76: 3617-3620.
- Harding, P.R. (1968). Effect of ozone on Penicillium mold decay and sporulation, Plant Disease Rep., 52: 245-247.
- Heagle, A. S. and Strickland, A. (1972). Reaction of Erysiphe graminis f. sp. horedi to low level ozone. Phytopathology 62: 1142-1148.
- Hoigne, J., Bader, H. (1976). Role of hydroxyl radical Reactions in ozonetion processes in aqueous solutions. Water Res. 10: 377-386.
- Johnson, R. L., Curl. Bond, J. and Priboury, H. (1960). Method for Studying Soil microflora-Plant Disease Relationships. Burgess Publishing Co., Minneapolis, 178.
- Kells, S. A., Mason, L. J., Maier, D. E., Woloshuk,, C. P. (2001). fficacy and fumigation characteristics of ozone in stored maize. Journal of Stored Products Research 37 (2001) 371–382.
- Kendrick, B. (1971). Taxonomy of fungi Imperfect. Toronto university, Canada.
- Kim, J. G., Yousef, A. E. and Dave, S. A. (1999). Application of ozone for enhancing the microbiological safety and Quality of foods: a review. Journal of Food Protection, 62: 1071-1087.
- Klick, M.A. and Pitt. J.I. (1988) A laboratory guide to common Aspergillus species and their teleomorphs. Published by Commonwealth Scientific and Industrial Research Organisation, Division of Food Processing. North Ryde, NSW Australia. 116 pp.
- Komanapalli, I R. and Lau, B.H.S. (1996). Ozone induce damage of Escherichia coli k-12. Applied and Environmental Microbiology 46:610-614.
- Krause, C. R., Weidensaul, T. C. (1978). Effects of ozone on the sporulsation, germination and pathogenicity of Botrytis cinerea. Phytopathology, 68: 195-197.
- Liew, C. L. and prange, R.K. (1994). Effect of ozone and storage temperature on postharvest diseases and physiology of carrots (Daucus carota L.). Journal of American Society of Horticulture Science, 119: 563-597.
- Magnoli, C., Violante, M., Combina, M., Palacio, G., Dalcero, A. (2003). Mycoflora and ochratoxin-producing strains of Aspergillus section Nigri in wine grapes in Argentina. Letters in Applied Microbiology 37: 179–184.
- Margosan, D. A. and Smilanick, J. L. (1998). Mortality of spores of Botrytis cinerea, Monilinia fructicola, Penicillium digitatum, and Rhizopus stolonifer after exposure to ozone under humid conditions. Phytopathology, 88: 58.
- Mason, L.J., Woloshuk, C.P., Maier, D.E. (1997). Efficacy of ozone to control insects, molds and mycotoxins. In: Donahaye, E.J., Navarro, S., Varnava, A. (Eds.), Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products. Nicosia, Cyprus Printer Ltd., Nicosia, pp. 665–670.
- Ministry of Agriculture and Water (1996). Agriculture Statistics Year Book, vol 9. Department of Economics Studies and Statistics.
- Morin, A., Moresoli, C.. Rodrique, N., Dumont, J., Racine, M. and Poitras, E. (1993). Effects of carbon, nitrogen and agitation on exopoysaccharide production by Enterobacter agglomerans grown on low-grade maple sap. Enzyme Microbiology and Technology. 15: 500-507.
- Moubasher, A. H. (1993). Soil fungi in Qatar and other Arab countries. Scientific and Applied Research Center, university of Qatar. pp. 566.
- Najafi, M. B. H. and Khodaparast, M.H.H. (2009). Efficacy of ozone to reduce microbial populations in date fruits. Food Control 20: 27–30
- Nassar, M.S.M. (1986). Mycoflora associated with dates in Aswan area.
- M.S. Thesis. Bot. Dept., Fac. Sci., Assuit Univ., Egypt.
- Palou, L., Smilanickb, J.L., Crisosto, C.H., Mansour, M., Plaza, P. (2003). Ozone gas penetration and control of the sporulation of Penicillium digitatum and Penicillium italicum within commercial packages of oranges during cold storage of oranges. Crop Protection 22:1131–1134
- Prat, R., Nofre, C. and Cier, A. (1968). Effects de I’hypochlorite de sodium de I’ozone et des radiations ionisontes dur les constituents pyrimidique de’Escherichia colia. Ann. Inst. Pasteur Paris 114:595-607.
- Pitt, J.I. (1979). The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press London, New York, Sydney
- Raper, K. and Fennell, D. I. ( 1965). The Genus Aspergillus. Willians and Wilkins Co. Baltimore.
- Robert, A.S., Hoekstra, Frisvad, J.C., Filtenborg, O. (1996) Introduction to food-borne fungi. Printed by Ponsen and Looyen, Wageningen, The Netherlands.
- Romero, S.M., Comerio, R.M., Larumbe, G., Ritieni, A., Vaamonde, G., Fernandez Pinto, V. (2005). Toxigenic fungi isolated from dried vine fruits in Argentina. International Journal of Food Microbiology 104: 43– 49
- Samson, R. A. (1979). A compilation of the Aspergillus described since 1965. C. B. S. Stud. Mycol. 18.
- Sarig, P., Zahavi, T., Zutkhi, Y., Yannai, S., lisher, N., and Ben-Arie, R. (1996). Ozone for control and post harvest decay of table grapes cause by Rhizopus stolonifer. Physiology and Molecular Plant Pathology, 48: 403-415.
- Scott, D. B. M. ( 1975). The effect of ozone on nucleic acids and their derivatives. P. 226-240. in W. j. Blogoslawski and R.G. Rice ( ed.), Aquatic applications of ozone. International Ozone Institute, Syracuse, N.Y.
- Xu, P., Janex, M. L., Savoye, P., Cockx, A., & Lazarova, V. (2002). Waste water disinfection by ozone: Main parameters for process design.Water Research, 36: 1043-1055.

This work is licensed under a Creative Commons Attribution 4.0 International License.





