How to Cite | Publication History | PlumX Article Matrix
L. M. S. Rudrammaji1, Veena. G. Sonole1, M. S. Dinesh2 and Sumasridhar3
1Department of Biotechnology, Sri Jayachamarajendra College of Engineering, Mysore - 570 006 India.
2Department of Biotechnology, PES Institute of Technology, 100 Feet Ring Road, BSK III Stage, Bangalore - 560 085 India.
3Department of Biochemistry, Dayananda Sagar College of Dental Sciences, Kumaraswamy Layout, Bangalore - 560 078 India.
ABSTRACT: The effect of coelomic fluids from Eisenia foetida and Perionyx excavatus on coagulation and hemolysis were examined separately. Coelomic fluids from Eisenia foetida and Perionyx excavatus were extracted separately from heat shock treatment. The coelomic fluid from Perionyx excavatus showed procoagulant and hemolytic activities whereas the coelomic fluid from Eisenia foetida exhibited only procoagulant activity and not hemolytic activity. These results clearly suggests that these two earthworm species have different factors responsible for the different pharmacological activities in their coelomic fluids.
KEYWORDS:
Coelomic fluid; procoagulant activity; hemolytic activity; Eisenia foetida; Perionyx excavatus
Download this article as:| Copy the following to cite this article: Rudrammaji L. M. S, Veena, Sonole G, Dinesh M. S, Sumasridhar. Procoagulant and Hemolytic Activities of Coelomic Fluid of Earthworms Eisenia Foetida and Perionyx Excavatus. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Rudrammaji L. M. S, Veena, Sonole G, Dinesh M. S. Sumasridhar. Procoagulant and Hemolytic Activities of Coelomic Fluid of Earthworms Eisenia Foetida and Perionyx Excavatus. Biosci Biotechnol Res Asia 2009;6(1) . Available from: https://www.biotech-asia.org/?p=8531 |
Introduction
The body fluid filled in the space between body wall and gut is called coelomic fluid (CF). The coelomic fluid of earthworms contain various molecules which exhibit important biological properties such as antimicrobial substances1-4, hemagglutinating5 and hemolytic agents6. The primary function of the CF presumably, is to destroy membranes of foreign cells, a mechanism that causes cell death by cytosol release and is attributed to coeolomocytes which secrete humoral effectors into the coelomic fluid. The components of the CF are lectin in character7-9.
The present study reveals the comparative characterization of CFs of Eisenia foetida and Perionyx excavatus with respect to their effect on coagulation and hemolysis.
Materials and Methods
Perionyx excavatus and Eisenia foetida were obtained from GKVK (Gandhi Krishi Vignyan Kendra) Bangalore, and were cultured. Uniplastin kit was purchased from Tulip Diagnostics (P) Ltd. Goa. Human blood samples were from healthy volunteers. All other chemicals were of analytical grade purchased from S.D. fine chemicals and HiMedia laboratories, Mumbai, India.
Culturing of Earthworms
Eisenia foetida and Perionyx excavatus were cultured on suitable bedding in plastic trays. Under ideal conditions, they were fed with organic substances from plant and animal origin. The feed stock chosen were dairy and beef manures which is considered as best natural food for the Earthworms10. 75%- 80% moisture content were maintained so that the average worm weight and the reproduction rate increased11. As the worms are aerobic, sufficient aeration was provided.
Extraction of Coelomic fluid
Extraction of Coelomic fluid from chosen earthworm species was performed according to the method of Kale R., (1991)12. The earthworms were washed with cold water 3-4 times at room temperature and their body surface were dried on filter paper. Then they were subjected to heat shock. Heat shock was performed by using 35°C – 40°C water in suitable container which was rubbed against the body of the earthworms. The procedure is repeated for 3- 4 times. The coelomic fluid obtained from the body of the earthworms was then collected and stored in vials.
Estimation of protein
Protein was measured by the method of Lowry et al., (1951)13 using bovine serum albumin standard (0-75µg).
Assay of Hemolytic activity
Preparation of washed erythrocytes
Human blood was collected from the vein of healthy volunteers who had not taken any medication for at least 2 weeks and were non-smokers. 9 volumes of blood were collected into 1 volume of acid citrate dextrose. Centrifugation was performed at 5000rpm for 10min. Supernatant was discarded and the pellet (packed erythrocytes) was washed in phosphate buffered saline (PBS) two times and used for the assay.
b) Assay of hemolytic activity
To assay direct hemolytic activity, the washed erythrocytes were suspended in 9 volumes of PBS and 1ml of suspension was incubated separately with 60µg/ml of CF from Eisenia foetida and 105µg/ml of CF from Perionyx excavatus at 37°C for 30min. The reaction was stopped by adding 10ml of ice cold PBS and centrifuged at 4°C for 10min at 800rpm. The amount of hemoglobin released in the supernatant was measured at 540nm. The experiment was repeated three times. For control, to 1ml of packed erythrocytes 9 volumes of water was added and the result was accounted as 100% hemolysis14.
Coagulation Assay
a) Preparation of plasma
Human blood was collected from the vein of healthy volunteers who had not taken any medication for at least 2 weeks and were non-smokers. 9 volumes of blood were collected into 1 volume of acid citrate dextrose. Centrifugation was performed at 5000rpm for 10min. The supernatant collected was plasma.
b) Assay of Prothrombin time
Assay for coagulation is based on prothrombin time. For concentration dependent assay, different concentrations (10µg/ml – 60µg/ml) of protein sample was added to 0.1ml of plasma. Incubation was done for 10min. 0.2ml of prothrombin reagent was added after the incubation. The time taken to form a clot in each case was recorded. For time dependent assay, the clot formation was recorded using a protein concentration of 40µg/ml at different time intervals (2min – 30 min). The experiment was repeated three times.
Results and Discussions
The CF of Perionyx excavatus exhibited hemolytic activity on washed intact erythrocytes. The activity was concentration dependent whereas CF of Eisenia foetida did not exhibit any hemolytic activity on washed erythrocytes. This result is in constrast with the result obtained from earlier workers6 (Sven Lange et al., 1999). This may be due to geographical variations in the contents of CFs from the same species. The results revealed the absence of hemolytic factors responsible for the hemolytic activity in the CF of Eisenia foetida and their presence in CF of Perionyx excavatus. The hemolytic activity of CF of Perionyx excavatus was concentration dependent as shown in the fig. 1.
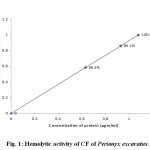 |
Figure 1: Hemolytic activity of CF of Perionyx excavatus.
|
(Each value represents mean of three independent determinations)
Coagulation assay was performed to the CFs of Eisenia foetida and Perionyx excavatus. Both the coelomic fluids were found to be procoagulants. The prothrombin time was dependant on concentration of protein (fig.2 and fig.3) and incubation time (fig.4 and fig.5). The results clearly indicate that with the increase in concentration of the protein, the time taken to form clot decreased clearly indicating the procoagulant activity.
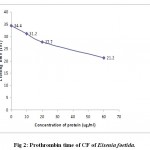 |
Figure 2: Prothrombin time of CF of Eisenia foetida.
|
(Each value represents mean of three independent determinations)
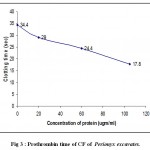 |
Figure 3 : Prothrombin time of CF of Perionyx excavatus.
|
(Each value represents mean of three independent determinations)
With 40µg/ml protein in CF of Eisenia foetida and 80µg/ml protein in CF of Perionyx excavatus, prothrombin time was recorded at different incubation time. An increase in the incubation time resulted in a decrease in the clotting time (fig.4 and fig.5). The results indicate that the procoagulant potency of CF of Eisenia foetida is slightly more than that of the CF of Perionyx excavatus.
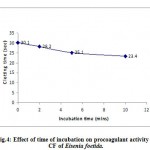 |
Figure 4: Effect of time of incubation on procoagulant activity of CF of Eisenia foetida.
|
(Each value represents mean of three independent determinations)
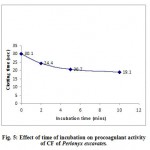 |
Figure 5: Effect of time of incubation on procoagulant activity of CF of Perionyx excavatus. |
(Each value represents mean of three independent determinations)
The hemolytic and procoagulant activities may be due to different factors present in the CF of different species of earthworms as the CF of Eisenia foetida exhibited procoagulant activity but not hemolytic activity whereas the CF of Perionyx excavatus exhibited both the activities. It also reveals the fact that different species of earthworms may comprise different molecules.
References
- Valembois P., Roch P., Lassegues M and Cassand P, 1982. Antibacterial activity of the hemolytic system from the earthworm Eisenia fetida Andrei, J. Invertebr. Pathol. 40, 21-27.
- Lassegues M., Roch P and Valembois P, 1989. Antibacterial activity of Eisenia fetida Andrei coelomic fluid: Evidence, induction and animal protection, J. Invertebr. Pathol. 1-6.
- Milochau A., Lassegues M and Valembois P, 1997. Purification, characterization and activities of two hemolytic and antibacterial proteins from coelomic fluid of the annelid Eisenia fetida Andrei, Biochim. Biophys. Acta, 1337, 123-132.
- Weidong Pan., Zianghui Liu., Feng Ge and Tao Zheng, 2003. Reconfirmation of antimicrobial activity in coelomic fluid of the earthworm Eisenia fetida Andrei by colorimetric assay, J. Biosci. 28, 723-731.
- Mohrig W., Eue I., Kauschke E and Hennicke F, 1996. Cross reactivity of hemolytic and hemeagglutinating proteins in the coelomic fluid of lumbricidae (annelida), Comp. Biochem. Physiol. 115A, 19-30.
- Lange S., Kauschke E., Mohrig W and Cooper E. L , 1999. Biochemical characteristics of Eiseniapore, a pore forming protein in the coelomic fluid of earthworms, Eur. J. Biochem. 262, 547-556.
- Stein E., Wojdani A and Cooper E. L., 1982. Agglutinins in the earthworm Lumbricus terrestris: naturally and induced, Dev. Comp. Immunol. 6, 407-421.
- Wojdani A., Stein E., Lemmi C. A and Cooper E. L., 1982. Agglutinins and proteins in the earthworm Lumbricus terrestris before and after injection of erythrocytes, carbohydrates and other materials, Dev. Comp. Immunol. 6. 613-624.
- Stein E and Cooper E. L., 1983. Carbohydrate and glycoprotein inhibitors of naturally occurring and induced agglutinins from the earthworm Lumbricus terrestris. Comp. Biochem. Physiol. 76B, 197-206.
- Gaddie R. E and Douglas D.,E., 1975. Earthworms for ecology and profit, Vol 10, Scientific earthworm forming. 180pp.
- George, 2004, Feasibility of developing the organic and transitional form market processing municipal and form organic wastes using large scale vermin composting. Good earth organic resources group, http//www.alternativeorganic.com.
- Kale R., D., 1991. Vermiculture: scope for new Biotechnology. In: Earthworms resources and vermiculture. (Ed. Director, ZSI, Calcutta, India). 105-108.
- Lowry O.H., Rosebrough N.,J, Farral and Randall R.,J, 1951. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 193, 265-275.
- Rudrammaji L., M., S and Gowda T., V, 1997, Purification and characterization of three acidic, cytotoxic phospholipases A2 from Indian cobra (Naja naja naja) venom, Toxicon 36, 921-932.

This work is licensed under a Creative Commons Attribution 4.0 International License.





