How to Cite | Publication History | PlumX Article Matrix
Production of Galacto-Oligosaccharides by Marine Derived Fungus Aspergillus Flavus
A. Pavani, T. Prabhakar and G. Girija sankar
A. U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam - 530 003 India.
Corresponding Author E-mail: pavani71@gmail.com
ABSTRACT: The aim of this research is to develop a cost effective method for the synthesis of oligosaccharides by using Aspergillus flavus (MTTC 9349) screened from marine fungus. This study was carried out, with a view of the marine strains may have better potential than their terrestrial counterparts. This is due to essential nutrients provided by marine biotopes for nurturing the isolates, and to extreme environmental niches. The marine fungi and their by-products may have potential values in food processing, fermentation, pharmaceutical and biopolymer industries. In this project we developed a production process of galacto-oligosaccharides (GOS), also known as Bifidus growth factor, are produced from lactose. The synthesis of GOS carried out by permeabilized and mutated cells of A. flavus. The process was optimized with different chemical agents for permeabilization; at substrate (lactose) concentrations ranging from 0.5 M to 2.25 M and pH.
KEYWORDS: A. flavus; Marine derived fungus; b-galactosidase; GOS. Bifidus factor
Download this article as:| Copy the following to cite this article: Pavani A, Prabhakar T, sankar G. G. Production of Galacto-Oligosaccharides by Marine Derived Fungus Aspergillus Flavus. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Pavani A, Prabhakar T, sankar G. G. Production of Galacto-Oligosaccharides by Marine Derived Fungus Aspergillus Flavus. Biosci Biotechnol Res Asia 2008;6(1) . Available from: https://www.biotech-asia.org/?p=8075 |
Introduction
The term “oligosaccharides” is broadly used for saccharides having the degree of polymerization of 2–10 (Nakakuki 1993). Structurally, Galacto oligosaccharides are composed of 2–20 monosaccharide residues linked by glycosidic bonds that are readily hydrolyzed to their constituent monosaccharide’s either by acids (chemical) or by specific enzyme beta galactosidase (Prenosil et al 1987) (enzymatic). The enzymatic synthesis of GOS from lactose has been reported by many authors (Tzortzis, Goulas, Gibson, 2005; Onishi, & Tanaka1998; Wierzbicki & Kosikowski 1972; Cruz et al 1999). The concentrations and structures of the oligosaccharides produced are mainly dependent on the concentration of substrate (Boon et al, 2000). GOS stimulate the proliferation of lactic acid bacteria and bifidobacteria in the human intestine (Sako, Matsumoto, & Tanaka, 1999).
In this paper, a study on the enzymatic production of GOS from lactose by immobilization of whole cells of A. flavus was reported. The chemical synthesis of GOS requires multiple protection and de-protection steps (Sears & Wong, 2001). This complexity does not render this synthetic route attractive for industrial applications. To overcome this limitation, new strategies for the production of GOS by various methods of immobilizations were used (Aziz Tanriseven, S¸enay Dogan et al 2002). Purification of enzyme is expensive process, so it is desired to develop a new inexpensive method of production of GOS by using whole cells. The crude enzyme also performed well (Barbara Splechtna et al 2006) for the production of GOS to avoid expensive purification process. (Tzortzis, Goulas & Gibson, 2005) Whole cells of a novel strain, Bifidobacterium bifidum was used for the production of GOS.
Marine derived fungus A. flavus which is proven for the production of b-galactosidase, therefore it of interest to find whether the fungus A. flavus can produce GOS comparable to the GOS produced by the pure enzyme immobilization. In this study we try to find out the optimum conditions for the production of GOS from lactose by mutated cells of A. flavus.
Mechanism of action
In the enzymatic hydrolysis of lactose, the enzyme b-galactosidase transfers a galactose moiety to an acceptor containing a hydroxyl group. If this acceptor is a water molecule, galactose is liberated (hydrolysis). When a sugar moiety acts as the acceptor, galactosyl-oligosaccharides (GOS) are formed. (Fig.1) In addition to hydrolysis, b-galactosidase from various micro organisms is also known to catalyze trans-galactolysation reactions. The formed galactosyl-oligosaccharides vary in chain length and in the inter connection of the monomer units (Crittenden & Playne1996).
GOS are usually considered as non-digestible, mildly sweet, poorly viscous, water-soluble sugars. They are functional food ingredients with a great potential to improve the quality of many foods. GOS have various physiological functions, such as the improvement of the intestinal microflora based on the selective proliferation of Bifidobacteria and the stimulation of mineral absorption.
Flow diagram for the production of Galacto-oligosaccharides
Materials and methods
Chemicals
Lactose monohydrate was supplied from Merck; 2-nitrophenyl- β-D-galactopyranoside was procured from Carbosynth Limited, Berkshire, U.K. All other chemicals and medium constituents in this study were of analytical grade and procured from Sigma-Aldrich and s.d fine chemicals.
Microorganism
The Aspergillus flavus derived from marine fungus collected at Bay of Bengal near Vishakapatnam in August 2005, using dilution-plate method (Christensen 1963) on PDA medium (Nielsen & Sorensen 1997). It was identified according to its morphological characteristics and confirmed by Prof. Ananthpadnabhan (IMTECH, Chandigarh). Working stocks were prepared on Potato Dextrose Agar slants and stored at 40 C. A. flavus was immobilized in calcium alginate and used for the production of GOS.
Enzymatic synthesis of GOS
Lactose solutions were prepared in different concentrations ranging from 0.5 M to 2.25 M in 50 mM potassium phosphate buffer containing 1 mM MgCl2. Different reaction conditions were assayed: temperature (250, 370, 400, and 50 0C), pH (4.5, 5.5, 6.5, 7.0, 7.5, 8.0), Immobilized Aspergillus flavus cells 2%. Experiments were carried out in duplicate. Reactions were performed in EM flasks incubated in an orbital shaker at 200 rpm. Samples were withdrawn at specific time intervals (30, 60, 120, 180, 240, and 300 min) and immediately immersed in boiling water for 5 min to inactivate the enzyme. The samples were stored at 40 C for subsequent analysis.
Chromatographic determination of carbohydrates
The samples were analyzed by HPLC using amino column (Shodex Asahipak NH2P-50 4E) at room temperature. The column was eluted with acetonitrile : water (80 : 20) at a flow rate of 1 ml/min. The peaks were detected by refractive index detector and it was assumed that the response was independent on the degree of polymerization. The amount of each saccharide was calculated from the area of corresponding peak. The number of sugar units of the oligosaccharides was found on the basis of retention time, trisaccharides and tetrasaccharides were identified on the basis of having a retention time later than of disaccharides. Lactose, glucose, galactose, and higher saccharides, such as tri- and tetrasaccharides were measured as weight percentage.
Samples and standard solutions of glucose, galactose, and lactose were filtered through a Millipore (0.45 micron) membrane before injection in to HPLC column. Twenty micro liters were injected using an auto sampler and separations were performed at a flow rate of 1 ml/min. Quantification of each sugar was performed by external calibration using standard solutions of galactose, glucose, lactose. The yield of GOS was expressed as percentage of carbohydrate weight formed per weight of lactose initially present. The peaks were measured with refractive index detector with the sample retention time 30 minutes.
Fig1.0. HPLC chromatogram: carbohydrate profile obtained from lactose hydrolysis produced by immobilization of A. flavus at pH 7.00, 400 C, 2.25 M of lactose solution. The identified compounds are indicated: (1) galactose, (2) glucose, (3) lactose, and (4, 5 and 6) Galacto oligo saccharides.
Results and discussions
Permeabilization of A. flavus with different chemical agents
Major part of enzyme β-galactosidase in cells of A. flavus was intracellular. So treatment with different chemical agent leads to permeabilization of cell wall there by the enzyme β-galactosidase, accessible with the substrate. Among various chemical agents (Fig 1.1) ethanol and toluene were found to be best permeabilizing agents for A. flavus followed by acetone.
 |
Figure 1: Enzymatic reaction of lactose to Galacto oligosaccharides and glucose.
|
Permeabilization with Detergents
Among different detergents of cationic, nonionic, anionic were tried. (Fig 1.2) Out of which Cetyltrimethyl ammonium bromide shows best permeabilizing activity of the mutated cells of A. flavus. Various concentrations of CTAB ranging from 1.37×10-3 M to 1.09×10-2 M concentrations were tried. The cells of A. flavus after treatment with CTAB of 2.75×10-3 M concentration (Fig 1.3) yields better β-galactosidase activity compare with the other organic solvents and detergents.
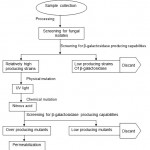 |
Figure 2: Flow diagram for the production of Galacto-oligosaccharides.
|
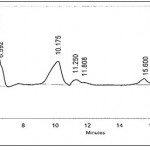 |
Figure 3
|
Effect of substrate concentration on production of GOS
Mutated and permeabilized cells of A. flavus were immobilized in sodium alginate and used for the production of GOS. For initial lactose concentrations of 0.5M, 0.75M, 1M, 1.25M, 1.5M, 1.75M, 2M and 2.25M were tried and the maximum GOS yields at 370 C were found to be 4.2 %, 6.4 %, 12.4 %, 16.7 %, 22.1%, 23.9 % after 160 minutes, respectively. Transgalactosylation significantly increased with the increased lactose concentrations (Fig 1.4).This is due to the fact that, at a low lactose concentration, hydrolysis is favoured since the amount of hydroxyl groups of carbohydrates is lower as compared to those of water, which act as acceptors of galactose.
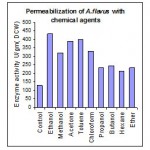 |
Figure 1a
|
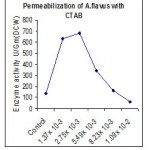 |
Figure 2b
Click here to View figure |
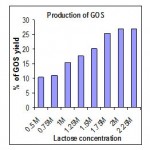 |
Figure 3c
|
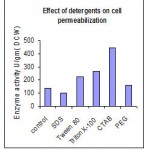 |
Figure 4d
|
Effect of pH on the production of GOS
We also investigated the effect of pH on the production of GOS by using wide range Mcilvaine buffers at pH 4.5 to 8.0 at lactose concentrations 1.75M. The decrease in the lactose concentration is rapid due to hydrolysis of lactose at pH 5.5, but the amount of GOS formed was higher at pH 7.00. (Table 1.0). β-galactosidase from either E. coli or S. cervacea reported that optimum pH for production of oligosaccharides were little higher than optimum pH for hydrolysis (Hubber et al 1976). Our findings are consistent with the reported data.
Reusability of immobilized A. flavus cells
Cells were reused up to 8 times for subsequent synthesis reactions with equal amounts of GOS being formed at analogous periods of time. From this point onwards, and always at the same reaction times, a slight decrease on the produced oligosaccharides was observed (compared with the initial eight reactions), resulting to a total 10% decrease in the synthesis of oligosaccharides after 12 times of re-use.
Table 1: Effect of pH on the production of GOS.
| % of saccharides | pH 4.5
|
pH 5.5 | pH6.5 | pH 7.0 | pH7.5 | pH 8.00 |
| Glucose | 16.2 | 39.4 | 41.6 | 35.2 | 33.2 | 21.6 |
| Glactose | 3.6 | 9.2 | 9.4 | 9.1 | 10.1 | 5.8 |
| Lactose | 70.7 | 39 | 32.5 | 34.9 | 40.4 | 59.3 |
| GOS | 9.4 | 12.3 | 16.6 | 20.8 | 16.3 | 13.3 |
Lactose solution (1.75 M) at different pH was incubated at 370 C with mutated cells of A. flavus at a concentration of 2% for 360 minutes.
Our results show that immobilization of whole cells was cost effective for the production of GOS. But the yield of GOS was comparatively low, with the immobilization of A.flavus. Immobilization of semi purified enzyme or crude extract also performed well in the production of GOS from lactose (Barbara Splechtna et al 2007). Degree of lactose conversion to GOS, mainly depend on the initial lactose concentration, the buffer (pH), salt concentration, temperature.
Conclusion
Based on the results reported in this study, β-galactosidase from A. flavus can be considered for the GOS production. To reduce enzyme costs, we successfully used the permeabilized A. flavus cells for the production GOS, thereby avoiding expensive purification of the enzyme. Moreover, the A. flavus cells can survive at 400 C in immobilized form causes, improved yield of GOS and decrease in microbial contamination.
Acknowledgements
The author is very thankful to Dr R.S. Prakasham, Indian Institute of Chemical Technology Hyderabad for providing necessary facilities for analysis of Galacto oligosaccharides and his valuable suggestions.
References
- Aziz Tanriseven , S¸enay Dogan et al., (2002). A novel method for the immobilization of b-galactosidase, Process biochemistry, 38, 27-30.
- Barbara Splechtna et al., (2007). Process development for the production of prebiotic galacto-oligosaccharides from lactose using β-galactosidase from Lactobacillus sp Biotechnol. J, 2, 480–485
- Boon, M. A., Janssen, A. E. M., & Van’t Riet, K. (2000). Effect of temperature and enzyme origin on the enzymatic synthesis of oligosaccharides. Enzyme and Microbial Technology, 26, 271–281.
- Christensen CM, (1963). Influence of small differences in moisture content upon the invasion of hard red winter wheat by Aspergillus restrictus and A. repens. Cereal Chem 40, 385-390.
- Crittenden, R. G., & Playne, M. J. (1996). Production, properties and applications of food-grade oligosaccharides. Trends in Food Science and Technology, 7, 353–361.
- Cruz, R., Cruz, V. A., Belote, J. G., Khenayfes, M. O., Dorta, C., Oliveira, L. H. S., et al. (1999). Production of trans-galactosylated oligosaccharides (TOS) by galactosyltransferase activity from Penicillium simplicissimum. Bioresource Technology, 70, 165–171.
- Czermak P, M. Ebrahimi, K. Grau, S. Netz, G. Sawatzki and P.H. Pfromm, (2004). Membrane-assisted production of galactosyl-oligosaccharides in a continuous process, J. Membr. Sci., 232 (1–2) 85–91.
- Ebrahimia M, Engela L, Petera S, Grau K, Czermak P, (2006). Membrane supported enzymatic synthesis of galactosyl-oligosaccharides. J. Desal., 200, 509-510.
- El-Shafei, H.A., and Rezkallah, L.A, (1999). Lactose degradation by β-galactosidase potential of Aspergillus flavus grown in salted whey, Bulletin of the National Research Center (Egypt) 24(3) 233-250
- Gaur, R., Pant, H., Jain, R., & Khare, S. K. (2006). Galacto-oligosaccharides synthesis by inmobilized Aspergillus oryzae β-galactosidase. Food Chemistry, 97, 426–430.
- Gibson G and Roberfroid M.B, (1995). Dietary modulation of the human colonic microbiology: introducing the concept of prebiotics, J. Nutrition, 125 ,1401–1412.
- Huber, R. E., Kurz, G., & Wallenfels, K. (1976). A quantitation of the factors which affect the hydrolase and transgalactosylase activities of β-galactosidase (E. coli) on lactose. Biochemistry, 15, 1994–2001.
- Iwasaki, K., Nakajima, M. and Nakao, S., (1996). Galactooligosaccharide production from lactose by an enzymic batch reaction using β-galactosidase. Process Biochemist, 31, 69-76.
- Kim, S. H., Lim, K. P., & Kim, H. S. (1997). Differences in the hydrolysis of lactose and other substrates by β-galactosidase from Kluyveromyces lactis. Journal of Dairy Science, 80, 2264–2269.
- Mahoney, R. R. (1998). Galactosyl-oligosaccharide formation during lactose hydrolysis: a review. Food Chemistry, 63, 147–154
- Mirdamadi, S. Moazami, N, Gorgani, N. (1997). Production of β-galactosidase in submerged media by Asp. oryzae , PTCC 5163 , J. Sci. IRI, 8(91), 23-28
- Nakakuki T. (Ed.). (1993). Oligosaccharides: Production, Properties and Applications, Japanese Technology Reviews, Vol. 3, No. 2, Gordon and Breach, Switzerland
- Nakayama, T., Amachi, T., β-Galactosidase, enzymology, in: Flickinger, M. C., Drew, S. W. (Eds.), (1999), Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation, John Willey, New York, pp. 1291–1305.
- Nielsen P, Sorensen J.(1997). Multi-target and medium independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus straind from barley rhizosphere. FEMS Microbiol Ecol 22, 183-192.
- Onishi, N., Tanaka, T., (1998), Galacto-oligosaccharide production using a recycling cell culture of Sterigmatomyces elviae CBS8119. Lett. Appl. Microbiol. 26, 136–139
- Onishi, N., Yamashiro, A., & Yokozeki, K. (1995). Production of galactooligosaccharide from lactose by Sterigmatomyces elviae CBS8119. Applied and Environmental Microbiology, 61(11), 4022–4025.
- Prenosil J.E., Stuker E., Bourne J.R. (1987). Formation of oligosaccharides during enzymatic lactose hydrolysis. 1. State of art. Biotechnol Bioeng 30:1019-1025,
- Sako, T., Matsumoto, K., & Tanaka, R. (1999). Recent progress on research and applications of non-digestible galacto-oligosaccharides. International Dairy Journal, 9, 69–80.
- Schoterman H.C, (2001). Galacto-oligosaccharides: properties and health aspects, in: B.V.Mc Cleary and L. Prosky (Eds.), Advanced Dietary Fibre Technology, Blackwell Science, 494–502.
- Sheu, D.C., Li, S.Y., Duan, K.J., Chen, C. W, (1998), Production of Galacto-oligosaccharides by β-galactosidase immobilized on glutaraldehyde treated chitosan beads. Biotechnol. Tech. 12, 273–276
- Tzortzis, G., Goulas, A. K., Gibson, G. R., (2005), Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl. Microbiol. Biotechnol., 68, 412–416.
- Wierzbicki, L. E., & Kosikowski, F. V. (1972). Formation of oligosaccharides during β-galactosidase action on lactose. Journal of Dairy Science, 56, 1400–1404.

This work is licensed under a Creative Commons Attribution 4.0 International License.





