How to Cite | Publication History | PlumX Article Matrix
Adhatoda Vasica: as a Potential Nematicidal Source Against Root-Knot Meloidogyne Incognita
Jashoda Kumari*, Ankita Sharma, Madhu Rathore and Kanika Sharma
Microbial Research Laboratory, Depertment of Botany, MLS University, Udaipur- 313001 India.
ABSTRACT: The effect of crude aqueous extract of different parts of Adhatoda vasica i.e. fresh leaves, inflorescence and bark on egg hatching and juvenile mortality of Meloidogyne incognita was studied in vitro. Fresh plant material was used to prepare the extracts. Observations were taken after 24, 48 and 72h. Leaf and inflorescence extract were found to be most effective on inhibition of egg hatching after 48h whereas bark extract was found to be less effective. 100% juvenile mortality was observed with undiluted crude aqueous leaf extract whereas inflorescence and bark extract exhibited only 86.66% and 52.66% mortality respectively after 48h.The preliminary phytochemical screening was done to show the presence of secondary metabolites in crude aqueous extract of Adhatoda vasica.
KEYWORDS:
Adhatoda vasica; Plant extract; Meloidogyne incognita; Hatching; Mortality
Download this article as:| Copy the following to cite this article: Kumari. J, Sharma. A, Rathore. M, Sharma. K. Adhatoda Vasica: as a Potential Nematicidal Source Against Root-Knot Meloidogyne Incognita. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Kumari. J, Sharma. A, Rathore. M, Sharma. K. Adhatoda Vasica: as a Potential Nematicidal Source Against Root-Knot Meloidogyne Incognita. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8921. |
Introduction
Disease of crop plants cause serious losses and have adverse effect on the agricultural economy of a country. Infection by nematode, especially Meloidogyne incognita (Root knot nematode) is one of the reason for severe losses in vegetable plants in all over world (Dawar, 2007). In recent years, the use of botanicals for the control of nematode is gaining importance because of increased awareness of environmental and human health hazards associated with the chemical control. Several plants are known to possess nematicidal and nematostatic properties (Hassan et al., 2000 and Pandey et al., 2001). Various organic additives of plant origin including oil- seed cakes, leaves and other plant parts are being effectively used for management of nematode (Patel et al., 2004; Sharma and Trivedi, 2002). In the present study, in vitro effect of crude aqueous extract of leaf, inflorescence and bark of Adhatoda vasica on egg hatching and larval mortality was studied. Preliminary phytochemical screening was also studied.
Material and method
Extract preparation:
Healthy leaves, inflorescence and bark of Adhatoda vasica used for the study. Fresh plant material was collected from Botanical garden, College of Science, Udaipur (Raj.) and washed with sterile distilled water. 100gm of respective fresh plant material was macerated with 100ml sterile distilled water and the resultant solution was allowed to stand for 48h in the refrigerator, after that the following solution was passed through a four ply muslin cloth and centrifuged for 15 minutes at 3000rpm. The supernatant was filtered through Whatman’s filter paper No.-1 (Patel et al., 2004) and filtrate was further diluted with sterile distilled water to prepare 5%, 10%, 20%, 40% and 80% extract concentrations. Undiluted filtrate was taken as 100% concentration.
Inoculum development
Brinjal seedlings were grown in steam sterilized soil and inoculated with pure stock culture of Meloidogyne incognita obtained from previous experiment. From galled roots egg masses of M. incognita were extracted after 45 days using sodium hypochlorite (Hussey and Barker, 1973) and incubated at 300C for 24h. The freshly hatched second stage nematode juveniles were collected and used as inoculum. The suspension containing juveniles of M. incognita in distill water was concentrated and standardized so that each 1ml suspension contained approximately 100 juveniles.
Nematicidal activity of extract
The nematicidal effect of plant extract was done by following two methods
Hatching assay
incognita egg masses of roughly similar sizes were placed in glass cavity blocks (1 egg mass/ cavity block) containing 3ml aqueous extract of respective plant and incubated at room temperature. Three replicates and a distilled water control were maintained simultaneously. Observations were taken at 24, 48 and 72h interval.
Mortality assay
Second stage juvenile (J2) of M .incognita were used to assay the effect of leaf, inflorescence and bark of A. vasica on juvenile mortality. One ml of nematode suspension containing approximately 100 M. incognita J2 juvenile was mixed with 3ml of respective plant extract in cavity blocks using sterile distilled water as control. Each treatment, including the control, was replicate 3 times. Observations were taken at 24, 48 and 72h intervals and percent larval mortality was calculated. Larval that did not respond to touch by a fine needle were counted as dead.
Phytochemical screening
Crude aqueous extract of A. vasica, leaf, inflorescence and bark was subjected to qualitative test for presence of alkaloids, sterols, tannins, carbohydrates and saponins suggested by Kokate et al., (1990)
Statistical analysis:
The significance of the work was analyzed statistically. R (Relation coefficient) and P-value was calculated by linear regression analysis using the software origin (6.0). Standard deviation and Standard error (SD & SE) were also calculated.
Result
Result of effect of crude aqueous extract of different plant parts of Adhatoda vasica on egg hatching and juvenile mortality of Meloidogyne incognita after 24, 48, and 72 h are listed in table 1 and 2. Since all plant parts inhibit egg hatching and showed juvenile mortality of M. incognita, it can be suggested that A. vasica possess nematicidal activity. At 24h, 28% egg hatching was observed in control, where as only 2.33 % egg hatching was observed with 100% concentration of leaf extract. Similarly, only 8.66% and 14.33% egg hatching was observed at the same concentration of inflorescence and bark extract after 24h. Results of mortality assay shows that maximum juvenile mortality was observed with leaf extract followed by inflorescence and bark extract. These results suggest that extract concentration and time of exposure are directly proportional to inhibition of egg hatching and juvenile mortality. Of the three plant parts used, aqueous extract of leaf was found to be most effective inhibitor of egg hatching followed by inflorescence and bark. These results are corroborated by statistical analysis. The experiment of inhibitory effect of egg hatching with leaf extract is significant (P= 0.02<0.05, R= 0.83) whereas with inflorescence and bark are not significant (R= -ve) Fig. (1, 2 & 3)
Table 1: Effect of crude aqueous extract of different plant parts of Adhatoda vasica on egg hatching of root-knot nematode Meloidogyne incognita.
| Plant part
|
Time duration
In hours |
Control
(%) |
% Egg Hatching | SD(±1) | SE(±1) | |||||
| 5% | 10% | 20% | 40% | 80% | 100% | |||||
| Leaf | 24 | 28 | 11.33
(59.53) |
8.33
(70.25) |
7.33
(73.82) |
6.33
(77.39) |
5.00
(82.14) |
2.33
(91.67) |
8.5 | 3.20 |
| 48 | 37 | 13.00
(64.86) |
12.66
(65.78) |
10.00
(72.97) |
9.66
(73.89) |
7.66
(79.29) |
5.66
(84.70) |
10.6 | 4.01 | |
| 72 | 47 | 17.00
(54.05) |
16.66
(64.55) |
14.66
(68.80) |
12.33
(73.76) |
9.33
(80.14) |
6.66
(85.82) |
13.5 | 5.09 | |
| Inflorescence | 24 | 28 | 19.00
(32.14) |
17.66
(36.92) |
16.33
(41.67) |
13.66
(51.21) |
9.66
(65.5) |
8.66
(69.07) |
6.5 | 2.46 |
| 48 | 37 | 22.00
(40.54) |
19.33
(47.75) |
17.33
(53.16) |
15.33
(68.06) |
11.33
(76.39) |
10.33
(78.48) |
8.9 | 3.39 | |
| 72 | 47 | 24.66
(47.53) |
21.66
(53.91) |
19.33
(58.87) |
17.33
(63.12) |
14.00
(70.21) |
12.66
(73.06) |
11.6 | 4.39 | |
| Bark | 24 | 28 | 27.00
(3.57) |
24.66
(11.92) |
23.33
(16.67) |
18.00
(35.71) |
16.66
(40.50) |
14.33
(48.82) |
5.3 | 2.02 |
| 48 | 37 | 31.66
(14.43) |
31.33
(15.32) |
27.66
(25.24) |
22.66
(38.75) |
18.66
(49.56) |
16.33
(55.86) |
7.5 | 2.85 | |
| 72 | 47 | 42.33
(9.93) |
38.66
(17.74) |
36.00
(23.40) |
28.66
(39.02) |
23.00
(51.06) |
20.00
(57.44) |
11.9 | 4.51 | |
Each value is an average of three replicates.
Value in ( ) parentheses are percent decreasing over control
Table 2: Effect of crude aqueous extract of different plant parts of Adhatoda vasica on juvenile mortality of root-knot nematode Meloidogyne incognita.
| Plant part
|
Time duration
In hours |
Control
(%) |
% Larval Mortality | SD(±1) | SE(±1) | |||||
| 5% | 10% | 20% | 40% | 80% | 100% | |||||
| Leaf | 24 | 00 | 44.66 | 58.33 | 63.00 | 70.33 | 82.66 | 96.00 | 30.9 | 11.71 |
| 48 | 00 | 54.66 | 64.66 | 68.00 | 79.33 | 88.00 | 100.00 | 32.4 | 12.24 | |
| 72 | 00 | 58.00 | 68.66 | 76.00 | 90.66 | 96.00 | 100.00 | 34.3 | 12.98 | |
| Inflorescence | 24 | 00 | 39.33 | 47.33 | 51.66 | 60.66 | 69.00 | 74.66 | 24.9 | 9.41 |
| 48 | 00 | 47.00 | 50.00 | 56.66 | 65.66 | 76.66 | 86.66 | 27.9 | 10.57 | |
| 72 | 00 | 48.66 | 55.33 | 63.00 | 70.33 | 83.66 | 89.66 | 29.7 | 11.22 | |
| Bark | 24 | 00 | 00 | 27.00 | 32.33 | 40.66 | 43.33 | 49.33 | 20.1 | 7.61 |
| 48 | 00 | 14.66 | 30.33 | 37.66 | 44.00 | 47.33 | 52.66 | 18.9 | 7.17 | |
| 72 | 00 | 20.00 | 34.66 | 46.00 | 47.66 | 51.66 | 59.33 | 20.7 | 7.82 | |
Each value is an average of three replicates.
Table 3: Preliminary phytochemical screening of Adhatoda vasica.
| Plant part | Alkaloid | carbohydrate | Flavonoid | Volatile oil | Tannin | Saponin | Steroid |
| Leaf | + | – | – | + | + | + | + |
| Inflorescence | + | – | – | + | – | + | – |
| Bark | – | – | – | – | + | + | – |
+ Present, – absent
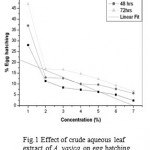 |
Figure 1: Effect of crude aqueous leaf extract of A. vasica on egg hatching of M. incognita. (R=0.832; P= 0.020).
|
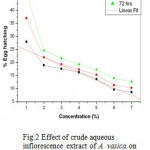 |
Figure 2 Effect of crude aqueous inflorescence extract of A. vasica on egg hatching of M. incognita. (R= -0.954; P= 8.26).
|
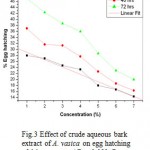 |
Figure 3: Effect of crude aqueous bark extract of A. vasica on egg hatching of M. incognita. ( R= -0.983; P= <0.0001).
|
In case of juvenile mortality experiment, significant P values were obtained for the crude aqueous extract of all three plant parts. Higher value of R (Correlation coefficient) in linear regression plots gave good linearization between concentration of extracts and percent mortality rate of M. incognita. (Fig 4, 5 & 6) Preliminary phytochemical screening are presented in Table no. 3
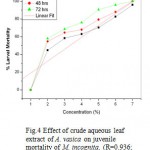 |
Figure 4: Effect of crude aqueous leaf extract of A. vasica on juvenile mortality of M. incognita. (R=0.936; P= 0.0019).
|
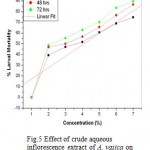 |
Figure 5: Effect of crude aqueous inflorescence extract of A. vasica on juvenile mortality of M. incognita. (R=0.923; P= 0.0029).
|
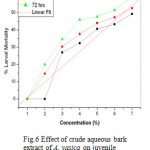 |
Figure 6: Effect of crude aqueous bark extract of A. vasica on juvenile mortality of M. incognita. (R=0.950; P= 0.0010).
|
Discussion
Plants are a source of naturally occurring pesticides. Plants contain several secondary metabolites which are responsible for their antimicrobial activity (Mcgow anf Eloff, 2005; Baswa et al., 2001). Most of these secondary metabolites like alkaloids, flavonoids, saponins, tannins volatile oil etc. are soluble in water as well as organic solvent (Harborne,1984). Nematicidal activity of aqueous extract of several plants has been reported earlier (Singh et al., 2001; Sharma and Patel 2001; Sosamma and Jayasree, 2002). In present study significant larval mortality and inhibition of egg hatching was observed with crude aqueous extract of leaf, inflorescence and bark of A. vasica. The presence of some toxic chemical compounds having the nematicidal/ nematotoxic activity may be responsible for the larval mortality and inhibition of egg hatching (Shngh and Sitaramaiah, 1973; Khan 1974). Several nematicidal compounds like alkaloids, flavonoids, terpinoids and phenolic compounds have been isolated from plants (David J. Chitwood, 2002). The nematicidal activity of aqueous extract of A. vasica is due to presence of biologically active compounds. Wide spectrum of antimicrobial activity such as antibacterial, antifungal activity, pesticidal activity etc (Prema, 2004; Inee Gogai et al., 2005; Sadek, 2003) of A. vasica are reported. Results of preliminary phytochemical screening also suggest the presence of alkaloids, volatile oils saponins, tannins and steroid in A. vasica. Thus it can be suggested that inhibition of hatching and juvenile mortality of M. incognita may be attributed to the presence of these secondary metabolite in the crude aqueous extract of A. vasica.
Reference
- Baswa M Rath C C, Dash S K and Mishra R K 2001 Antibacterial activity of Karanj (Pongamia pinnata) and Neem (Azadirachta indica) seed oil; a preliminary report. Microbs 105 183-189
- David J Chitwood 2002 Phytochemical based strategies for nematode control; Annu. Rev. Phytopathol 40 221-249
- Dawar S, Younus S M And Zaki M J 2007 Use of Eucalyptus sp, In the control of Meloidogyne javanica root- knot nematode; Pak. J. Bot. 39(6): 2209-2214
- Harborne J B 1984 Method of plant analysis, In: Phytochemical methods , Chapman and Hall, London, New Yark, pp 05-06
- Hassan S M E, Rahman M Sq, Amin M R, Majumdar U K and Ahmad M U 2000 Effect of Neem (Azadirachta indica) on the root-knot nematode (Meloidogyne jananica ) of sweet gourd; Pak. J. Bio. Sci. 3(11) 1853-1854
- Hussey R S and Barker R K 1973 A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique; Plant Dis. Rep. 57 1025-1028
- Inee Gogoi, Rahman I, Dolui A K 2005 Preference of alternate host plants by Helopeltis theivora water house and their susceptibility to plant extract; Proc. of the National Acad. of Sci. India. section B, Biological Science; (vol. 75) (No. 3) 181-185
- Khan M W, Alam M M, Khan A M & Saxena S K 1974 Effect of water soluble fractions of oil cakes and bitter principles of neem on some fungi and nematodes; Acta Bot. Indica 2 120-128
- Kokate C K, Purohit A P and Gokhle S B 1990 Pharmacognocy. In Analytic pharmacogocy, 7th (Ed.) Nirali Prakashan, Pune; 122-124
- Mcgaw L J and Eloff J N 2005 Screening of 16 poisonous plants for antimicrobial, anthelmintic and cytotoxic activity in vitro; South African J. Bot. 71: 302-306
- Pandey R, Kalra A, Katiyar N and Kumar S 2001 Nematicidal activity in flowers of some medicinal and aromatic plants; Indial J. Nematol 31(1) 79-98
- Patel A D, Patel D J and Patel N B 2004 Effect of aqueous leaf extract of botanicals on egg hatching and larval penetration of Meloidogyne incognita in banana; Indian J Nematol 34(1) 37-39
- Prema P 2004 Antimicrobial activity of selected medicinal plants; Journal of Ecobiology (vol. 16) (No. 5) 333-337
- Sadek M M 2003 Antifeedant and toxic activity of Adhatoda vasica leaf extract against Spodoptera littoralis (Lep., Noctuidae); Journal of Applied Entomology vol. 127 pp 396-404 (9)
- Sharma D N P and Patel H R 2001 Effect of various exposure periods and concentrations of crude leaf extract of Bidi tobacco on egg hatching of root- knot nematode; Indian J. Nematol 31(2) 129-132
- Sharma N and Trivedi P C 2002 Screening of leaf extracts of some plants for their nematicidal and fungicidal properties against Meloidogyne incognita and fusarium oxisporium; Asian J. Exp. Sci. vol. 16, No. 122, 21-28
- Singh R A and Sitaramaiah K 1973 Control of plant parasitic nematode with organic amendments of soil; G.B. Pant University of Agric. & Tech. Pantngar, Research bulletin No. 6 pp 289
- Singh R, Chhabra H K and Kaul V K 2001 Effect of aqueous extract of plant products on hatching and penetration of Meloidogyne incognita infecting sunflower; Indian J Nematol 31(1) 34-37
- Sosamma V K and Jayasree D 2002 Effect of leaf extract on the mortality of root-knot nematode, Meloidogyne incognita juveniles; Indain J. Nematol. 32(2) 183-233.

This work is licensed under a Creative Commons Attribution 4.0 International License.





