How to Cite | Publication History | PlumX Article Matrix
N. Sivakumar1, S. Ponnusamy2 and P. M. Gopalsamy3
1Department of Chemistry, Chikkanna Government Arts College, Tiruppur, India.
2Department of Chemistry, Government Arts College (autonomous), Coimbatore, India.
3Department of Chemistry, Gobi Arts & Science College, Gobichettipalayam, India.
ABSTRACT: The adsorption of three different commercial dyes used in textile industry acid dye (Acid Blue 74), direct dye (Direct Black 19) and basic dyes (Basic Brown1) on a highly meso porous activated carbon obtained by single step steam pyrolysis of bio diesel industrial waste namely Jatropha -seed hull was investigated in terms of size of dye molecules, pore size and surface charge of the activated carbon. The activated carbon obtained from jatropha hull (JHC) was mainly meso porous in nature, with a surface area of 722 m2/g and an average pore diameter of 2.332 nm. CAC, the commercial reference was mainly micro porous with a surface area of 1026 m2/g and an average diameter of 1.8 nm. Small size molecules of acid dye and basic dye were adsorbed in high amounts on to both JHC and CAC. The amounts of direct dye, which is large in one or two dimensions of molecular structures, adsorbed on a mesoporous JHC was much higher than those on CAC. From these results it was clear that the adsorption of dyes on JHC activated carbon influenced greatly by pore size and surface charge of activated carbon. The results indicate that JHC could be employed as low-cost alternative to commercial activated carbon in wastewater treatment for the removal of acid dyes.
KEYWORDS: Activated carbon; jatropha husk; Adsorption; Porosity; Isotherms; surface area
Download this article as:| Copy the following to cite this article: Sivakumar. N, Ponnusamy. S, Gopalsamy. P. M. Characterization and Dye Effluent Treatment Applications of Mesoporous Activated Carbon Produced from Bio-Diesel Industrial Waste - Jatropha Hull. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Sivakumar. N, Ponnusamy. S, Gopalsamy. P. M. Characterization and Dye Effluent Treatment Applications of Mesoporous Activated Carbon Produced from Bio-Diesel Industrial Waste - Jatropha Hull. Biosci Biotechnol Res Asia 2009;6(2) . Available from: https://www.biotech-asia.org/?p=8989. |
Introduction
The economical decolourisation of textile processing effluents by removal of dyes remains as important problem although a number of techniques have been developed1.Activated carbon has been successfully used as an adsorbent for the removal of dyes from waste water. Activated carbon can be produced from a number of precursor materials including wood, agricultural wastes, coal, etc. the adsorptive properties of activated carbons are derived from their extensive internal pore structure, which is only present with a high surface area but also desired pore size distribution for the adsorption of molecular species with increasing ecological and economical significance of environmental protection, the use of waste biomass as feedstock material for the production of activated carbon is attracting. In the present study the feedstock materials namely jatropha husk residues which are produced by bio diesel industry2 in large quantities was subjected to pyrolysis. The prepared high surface area porous carbons are expected to be excellent adsorbents for the removal and recovery of high molecular compounds. From this point of view, in this work attempt was made to study the adsorption effect on the molecular size of the dyes at different parameters.
Materials and Methods
Preparation of activated carbon
The raw material, jatropha husk was collected, dried, pulverized and washed with water. The activation was performed by heating the sample in a muffle furnace using a purpose made stainless steel reactor at a rate of 3°C/min to 850°C for 2 hours under nitrogen gas atmosphere. When the activation temperature was reached, the N2 gas flow was switched to steam (100% H2O, 0.1g/min) and the sample was held at the specified temperature for predetermined activation time (40minutes). After activation the sample was allowed to cool in a nitrogen flow3 . The samples were grounded, sieved to 0.100mm mesh size and dried at 100.5°C for overnight before the determination of their chemical properties. Finally, the product was stored in a vacuum desiccator until required.
Adsorbent Characterization
The BET surface area of the activated carbon (SBET JHC) was obtained from N2 adsorption isotherm at 77K with sorpitometer [Smartsorb-91(India)]. Based on these data, the manufacturer’s software provides the Pore characteristics viz., the total pore volume (Vpore) by the BJH theory4 micro and meso size by both t – plot method and BJH method respectively5, 6. Determination of zero point charge (pHzpc)
The adsorbent (100mg ) suspension (particle size, 0.100mm) was prepared in 50ml solution of NaNO3 electrolyte of concentration, approximately 10⎯³ M. Aliquots of suspension were adjusted to various pH values with dilute NaOH and HNOз Solutions. After one-hour equilibration, the initial pH values were measured. Then 1.0 g of NaNOз was added to each aliquot to bring final electrolyte concentration to about 0.45M. After an additional one hour agitation, final pH was measured. The results were plotted with ∆pH {final pH – initial pH) against final pH. The pH at which ∆pH = 0 yielded pHzpc7.
Dye Characteristics
Acid dye (Acid Blue 74), direct dye (Direct Black 19) and basic dyes (Basic Brown 1) were obtained from Fluka, Clarinet and Sigma chemicals with approximately in 70% purity. The molecular structure and size of dye was calculated with molecular modeling system8. The estimated molecular structures and sizes in three dimensions of these dyes as shown in figure 1. All other chemicals were of AR grade and purchased from E. Merck (India). Double distilled water was used solution preparation. A Systronics UV-VIS spectrophotometer (160 A) was employed for absorbance measurements using cells of path length 1 cm.
Batch adsorption studies
Adsorption experiments were carried out by shaking adsorbents with 50 ml dye solution of required concentration and pH at 30°C in a thermo stated bacteriological incubator shaker operated at 200 rpm. The samples were withdrawn from the shaker and the dye solution was separated from the adsorbent by centrifugation. Dye concentration in the supernatant solution was estimated by measuring absorbance at maximum wavelength (λmax = 414 nm) and computing concentration from the calibration curve. Kinetics of adsorption was determined by analyzing adsorptive uptake of the dye colour from aqueous solution at different time intervals. Isothermal studies were conducted by adding various doses of adsorbent and shaking the reaction mixture for the equilibrium time. Influence of the pH was studied by adjusting the reaction mixture to different initial pH value and analyzing the residual colour for equilibrium contact time. The amount of dye adsorbed onto the carbons, Qe (mg/g), was calculated by mass balance relationship
Qe = V (Co– Ce) / W
Where Co and Ce are the initial and equilibrium liquid-phase concentrations of dye (mg/l), respectively, V the volume of the solution (l), and W the weight of the carbon used (g).
Results and Discussion
The porous properties of the activated carbon resulting from steam activation of jatropha hull calculated from the nitrogen adsorption-desorption isotherms. It is clear that JHC has an obviously larger Vmeso value than, and nearly the same Vmicro and SBET values as CAC. From table 1, the values of Dp indicate a characteristic of meso porous structure (i.e., pore diameter (2 nm < d < 50 nm) for JHC. . The results show that the large surface area and total pore volume of JHC is comparable with that of commercial activated carbons in the liquid phase adsorption. It is noted that total pore volume is usually used as an indication for the adsorption capacity, because its measurement is based on converting the amount of adsorbate (i.e., nitrogen) adsorbed at a relative pressure 0.975 to the volume liquid nitrogen. The amounts of Acid dye (Acid Blue 74) and basic dyes (Basic Brown 1), which exhibit small molecular weight as shown in Fig. 1, adsorbed on micro porous CAC is higher than that on meso porous JHC. This indicates that the specific surface area of activated carbon is an essential factor in adsorb in these acidic dyes because BET surface area of CAC is higher than that of JHC. On the other hand, the adsorbed amounts of Direct dye (Direct Black 19), which has relatively large molecular structure, were lower than those of others. These results suggest that pore size plays an important role rather than surface area in the adsorption of direct dyes with one or two large dimensions ,because JHC is meso porous and its average pore size is larger than the sizes of the dye molecules. On the relation between pore size and molecular sizes of adsorbates, Tamai et al.8 studied the adsorption of the dyes of relatively small size on activated carbons which have mainly micro and meso pores, and they suggested that the short-axis size of the dyes plays an important role in adsorption on micro porous activated carbons. According to the results, It is noteworthy that meso pores directly connected on the external surface of JHC, in which direct dye molecules are able to diffuse, contribute to the adsorption of direct dye (Direct Black 19). The amounts adsorbed on CAC and JHC increased with increasing dye concentration and the plateau value was higher on both CAC and JHC, compared with that of direct dye. The high adsorbed amounts of basic dyes could be interpreted in terms of the negative surface charge of the activated carbon (pHzpc) and the relatively small steric size of dye molecules as compared with the size of micro pore. That is, the electrostatic interaction acting between the surface of activated carbon and dye molecules, and the diffusion of dye molecules into not only meso pore but also micro pore increase the adsorbed amounts of basic dyes.
Table 1: Texture characteristics of activated carbons as determined from N2 adsorption at 77K.
| Source
|
SBET (m2/g) | Smic(m2/g) | Smeso(m2/g) | Vtotal(cm3/g) | Vmic(cm3/g) | Vmeso (cm3/g) | Average pore dia (nm) | pHzpc |
| CAC | 1026 | 630 | 396 | 0.598 | 0.361(60%) ` | 0.237(40%) ` | 1.8 | – |
| JHC | 722 | 210 | 512 | 0.418 | 0.117(28%) ` | 0.301(76%) | 2.332 | 5.8 |
 |
Figure 1: Molecular structures of a) Basic Brown 1 b) Acid Blue 74 c) Direct Black 19.
|
Successful application of the adsorption demands innovation of cheap, nontoxic, easily available adsorbents of known kinetic parameters and sorption characteristics. Foreknowledge of the optimal conditions would herald a better design and modeling of the process. Thus, the effect of contact time on the uptake of dyes on CAC and JHC were investigated from kinetic point of view. Preliminary investigations on the rate of uptake of dyes on CAC and JHC indicated that the processes are quite rapid and typically 50–60% of the ultimate adsorption occurs within the first hour of contact (Fig. 2). This adsorption subsequently gives way to a very slow approach to equilibrium and the equilibrium is achieved in 2–3 h in the case of JHC .This may be due to the fact that the activated carbon is composed of macro and micro pores. In the process of dye colour adsorption, initially dye molecule has to first encounter the boundary layer effect and then it has to diffuse from boundary layer film onto adsorbent surface and then finally it has to diffuse into the porous structure of the adsorbent9. It is suggested that the high adsorption of acid dye on JHC than CAC depends on the pore size and high meso pore volume of JHC in addition to specific surface area. That is, if acid dye has one large dimension in its molecular structure, the adsorption should change by the relation between one large dimension of dye molecule and pore size of activated carbon. From the figure 2 it is found that the time profile of dye uptake by both CAC and JHC is a single, smooth and continuous curve leading to saturation, suggesting the possible monolayer coverage of dye on the surface of the adsorbent10.
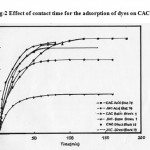 |
Figure 2: Effect of contact time for the adsorption of dyes on CAC and JHC.
|
Conclusion
It is reasonable to conclude from the present investigations on the adsorption of dyes on adsorbent prepared from industrial waste jatropha husk, carbonaceous adsorbent possessing organic nature and consequently having higher surface area with high porosity is efficient for the removal of dyes. The JHC is mainly meso porous in nature and acidic in character, while a commercial AC used as reference was found to be more micro-porous. The fitted Freundlich and Langmuir model suggests co-operative adsorption occurs in these narrow sized pores. Generally accessible micro pore areas showed significant adsorption which proved to be better in comparison to adsorption of commercially available micro porous activated carbon. The acidity of the carbon and the total surface area played complementary roles in promoting the adsorption of dyes. The adsorption of dyes on carbonaceous adsorbent is first order and pore-diffusion-controlled; and the carbonaceous adsorbent is about 80–90% as efficient as commercial activated carbon and thus can be used in its place for the removal of dyes from effluents in view of its cheaper cost.
References
- Adamson AW. Physical Chemistry of Surfaces. 5th ed. John Wiley and Sons, New York;
- 1990.
- Kandpal JB, Madan M. Jatropha curcus: a renewable source of energy for meeting future energy needs. Renewable Energy 1995; 6(2):159-60.
- Barrett EP, Joyner LG, Halenda PP. The determination of pore volume and area distribution in
- porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 1951; 73: 373-80.
- Michael Warhaurst A, Gordon L Mc Connachie, Simon J T Pollard. Characterization and applications of activated carbon produced from Moringa oleifera seed husks by single step pyrolysis. Wathes 1997; 31(4):759-66.
- Sing KSW, Ederett DH, W.Haul RA, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T. Reporting physisorption data for gas/solid systems with special reference to the determination
- of surface to the determination of surface area and porosity. Pure Appl.Chem 1985; 57:603 – 19.
- Satish M. Manocha, Vanraj B.Chauhan, Manocha LM. Studies on development of porosity in carbon from different types of bio wastes. Carbon Science 2002; 3 (1):1-5.
- Maria J. Martin, Adriana Artola M, Dolors Balaguer, Miquel Rigola. Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chemical Engineering Journal 2003; 94: 231–39.
- Hisashi Tamai, Takeshi Yoshida, Masahiko Sasaki, Hajime Yasuda. Dye adsorption on Meso porous activated carbon fiber obtained from pitch containing yttrium complex. Carbon 1999; 37: 983–89.
- Mohan SV, Rao NC, Karthikeyan J. Adsorption of direct azo dye from aqueous phase onto coal based sorbents: a kinetic and mechanistic study. J Haz Mat 2002; 90:189–04.
- Malik PK. Use of activated carbons prepared from sawdust and rice-husk for adsorption of Acid dyes: a case study of Acid Orange 10. Dyes and Pigments 2003; 56: 239–49.

This work is licensed under a Creative Commons Attribution 4.0 International License.





