How to Cite | Publication History | PlumX Article Matrix
Characterization of in Vivo Nitrate Reductase Activity in Triticum Vulgare Seedlings
Vinodh Ratnala1, Dsvgk Kaladhar2, B. Seshagiri3 and N. Sarada Mani4
1Department of Biotechnology, MAS Lab, NRC for Sorghum, Rajendranagar, Hyderabad India.
2Department of Bioinformatics, GITAM Institute of Science, GITAM University, Visakhapatnam India.
3Department of Biochemistry, M.V.R College of Post-Graduate Studies, Visakhapatnam India.
4Department of Botany, Andhra University, Visakhapatnam India.
ABSTRACT: Nitrate assimilation plays an important role in plant growth and bio-productivity. The first step of this process is the reduction of nitrate to nitrite in the cytosol. Investigations are undertaken to study the optimum conditions for assaying this enzyme in seedlings of Triticum vulgare. The in vivo NR activity in seven-day-old green seedlings increased linearly with the mass of the leaf tissue in the infiltration (assay) medium (5 ml total volume) up to 400 mg tissue. The optimum concentration of substrate, nitrate, in the external assay medium was 100 mM, the optimum assay pH was 7.5, with a decrease in enzyme activity in both acidic and alkaline ranges. The optimum assay temperature was 30°C with greatest inhibition of enzyme activity at 50°C (67%) at the end of 1 h exposure of leaf segments in the assay medium. Among the low molecular weight organic solvents added to the assay medium to enhance enzyme activity, 1% (v/v) n-butanol was the most effective causing 420% increase in enzyme activity. Where as 1%(v/v) acetone was the least potent organic solvent. 0.1% Triton X-100, a surfactant as well as a detergent, caused a 3.7 fold enhancement in NR activity over control.
KEYWORDS: In vivo characterization; Nitrate Reductase(NR) activity; Triticum vulgare
Download this article as:| Copy the following to cite this article: Ratnala. V, Kaladhar. D, Seshagiri. B, Mani. N. S. Characterization of in Vivo Nitrate Reductase Activity in Triticum Vulgare Seedlings. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Ratnala. V, Kaladhar. D, Seshagiri. B, Mani. N. S. Characterization of in Vivo Nitrate Reductase Activity in Triticum Vulgare Seedlings. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8837. |
Introduction
In natural environments the rate of plant growth and the productivity are delicately balanced with a number of nutritional factors. The supply of available soil nitrogen is one of the important factors and consequently nitrogen metabolism constitutes a major area of interest for improving plant productivity.
The two major sources of nitrogen for plant growth and bio-productivity are nitrate assimilation and biological nitrogen fixation 1,2. Though plants can utilize either ammonium or nitrate ions, nitrate is the predominant form of nitrogen available to most cultivated plants grown under normal field conditions. The protein accumulation in cultivated plants is largely influenced by the acquisition and reduction of nitrate nitrogen and its incorporation into the amino acids and proteins.
The reduction of nitrate to ammonia is catalyzed by two metallo-proteins, nitrate reductase and nitrite reductase. In the reduction of nitrate to nitrite, two main types of assimilatory nitrate reductases are distinguished: (a) ferredoxin dependent nitrate reductase occurring in cyanobacteria 3 and (b) Pyridine nucleotide – dependent nitrate reductase found in eukaryotic organisms 4,5,6,7,8. The reduction of nitrate to nitrite occurs in the cytosol 9. The enzyme nitrate reductase catalyzes this reaction:
NO¯+ NAD(P)H + H+ +NO2 e– ——> + NAD (P)+ +H2 O
Where NAD(P)H indicates NADH or NADPH.
The most common form of Nitrate Reductase(NR) uses only NADH as an electron donor. Another form of the enzyme that is found predominantly in non-green tissues such as roots can use either NADH or NADPH 10.
The NRs of higher plants are composed of two identical sub units, each containing three prosthetic groups: FAD (flavin adenine dinucleotide), heme, and molybdenum complexed to an organic molecule called a pterin 11
A model of the NR dimer, illustrating the three binding domains: molybdenum complex (M0C0), heme (cyt b 557), and FAD. The NADH binds at the FAD – binding region of each sub unit and initiates a two-electron transfer from the carboxyl (c) terminus, through each of the electron transfer components, to the amino (N) terminus. Nitrate is reducing at the molybolenum complex near the amino terminus 12.
Reduction of nitrate to nitrite, catalyzed by the enzyme nitrate reductase, has been considered the rate – limiting step in nitrogen assimilation 4. In the vegetative tissue of field-grown cereal crops, about 70 – 80% of nitrate reductase is localized in the leaves 12. A quantitative measurement of its activity could be helpful in assessing the amount of reduced nitrogen made available to the plant. Though significant correlation was observed between the amount of nitrogen supplied to the plant as estimated by the in vitro NR assay and the actual amount of nitrogen accumulated by the plant 12,13.14. The optimum conditions employed for the in vitro NR assay would seldom is obtained in situ. Also, the possibility of nitrate reductase inactivation by endogenous inhibitors 15 or proteases 16 during extraction of leaf tissue would lead to an under estimation of the in situ enzyme activity. Therefore, the use of in vivo NR assay, first developed by Kumada, H. 17 appears to be a desirable alternative. Studies were therefore conducted on the optimum requirements for obtaining a valid estimation of in situ NR activity in seedlings of Punjab Wheat.
Materials and Methods
Seed Material
Wheat (Triticum vulgare) seeds of Punjab variety were obtained from local seed stores.
Cultivation of Wheat Seedlings
Healthy seeds of wheat were surface – sterilized in 4% (v/v) sodium hypochlorite for 5 min, thoroughly washed several times in tap water and subsequently soaked for 3 hours in distilled water. The seeds were germinated in Petri dishes lined with a course filter paper (Kalpi) in distilled water in dark before being exposed to 20 mM KNO3 under a constant illumination of 5000 lux supplied by a bank of white, cool, fluorescent lamps.
Harvest of Seedlings
Unless otherwise stated, uniformly growing seven-day-old green seedlings were harvested at least 4 hours after exposure to light. For experiments involving etiolated seedlings, 3-day-old etiolated shoots raised in distilled water in dark were exposed to light and 20mM KNO3 simultaneously.
Induction of NR and assay of in vivo NR activity
NR was induced in green seedlings and etiolated shoots with 20mM KNO3 under constant illumination.
Unless otherwise mentioned, the standard, infiltration medium (2ml to 5 ml depending on the experiment) for the in vivo NR assay was composed of
— 100 mM KH2 PO4 – KOH, PH 7.5
— 100 mM KNO3
— 1 % butanol
— 0.1% Triton X – 100.
Leaf segments (1mm) equivalent to 0.1-0.2g fresh weight were incubated in dark at room temperature (300C) in 20ml glass vials with air-tight caps containing either 2ml (for greening shoots) or 5ml (for green leaf segments) infiltration medium. The contents were periodically shaken. After 1hour incubation, 0.2ml to 0.5ml of the infiltration medium was removed for nitrite analysis. The nitrate reductase activity was expressed as moles nitrite produced in hour/gram fresh weight.
Nitrite ( ) estimation
To nitrite solution or a known volume of the infiltration medium containing nitrite, 1ml of 1% (w/v) sulphanilamide reagent prepared in 3N HCL and 1ml of 0.02% (w/v) N – (1 – naphthyl) ethylenediamine dihydrochloride reagent were added in quick succession and the contents were thoroughly mixed. The colour was allowed to develop for 15 min prior to reading at 540nm in a UV-visible spectrophotometer (Systronics, Model 118).
The amount of nitrite formed was calculated based on
E1cm1mM Nitrite complex (540 nm) = 551mM
Results
In the present investigation, NR activity was assayed by the in vivo method in which leaf segments from NR induced seedlings were infiltrated in dark in a medium containing external nitrate. Nitrate from the external medium gets reduced in the leaf cell cytoplasm where NR is localized and the nitrite produced from nitrate reduction leaks into the external medium. For measurement of NR activity, this nitrite in the external infiltration medium is measured and the in vivo NR activity is expressed as m moles NO2– produced per hour per gram fresh weight.
The in vivo NR activity in 7-day-old green wheat seedlings increased linearly with the mass of the leaf tissue in the infiltration medium (Fig. 1). The linearity between the tissue mass and NR activity was maintained up to 400 mg of leaf tissue (Fig. 1).
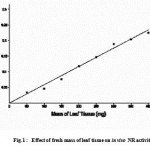 |
Figure 1 :Effect of fresh mass of leaf tissue on in vivo NR activity.
|
When the influence of external nitrate (substrate) concentration in the infiltration medium on the enzyme activity was investigated, NR activity was found to be dependent on the supply of exogenous nitrate in the assay medium. The optimum concentration was found to be 100 mM (Fig. 2). Higher concentrations did not result in an increase in enzyme activity. The results when plotted resulted in a hyperbolic curve (Fig.2).
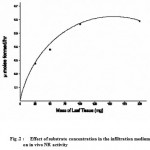 |
Figure 2 : Effect of substrate concentration in the infiltration medium on in vivo NR activity.
|
Subsequently, experiments were conducted to investigate the effect of assay pH on NR activity. The optimum assay pH for the enzyme activity was found to be 7.5, with a decrease in enzyme activity on either side of the optimum pH (Fig. 3).
 |
Figure 3: Effect of assay pH on in vivo NR activity.
|
The temperature optimum for the in vivo enzyme activity was found to be 300C with decrease in enzyme activity at lower and higher temperatures with maximum inhibition (67%) of the maximum observed at 500C incubation temperature (Fig.4))
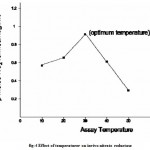 |
Figure 4: Effect of temperaturer on invivo nitrate reductase.
|
Since the in vivo NR activity was associated with membrane permeability, low molecular weight organic solvents and surfactant, Triton X-100, were employed to investigate their effect on in situ enzyme activity. Results in (Table 1) indicate that of the five organic solvents added to the incubation medium. 1% n – butanol was the most effective in enhancing enzyme activity over the control containing only buffered nitrate. The extent of enzyme activity in presence of 1% n-butanol was 4.2 – fold (420%) compared to control. Acetone was the least potent organic solvent as an additive to the enzyme assay medium.
In the presence of 0.1% Triton X-100 in the infiltration medium, there was 3.7 – fold (377%) improvement in the enzyme activity over the control. Compared to the enzyme activity in the presence of 0.1% Triton X-100, 1% n-butanol could cause only 2.2 – fold (219%) improvement in enzyme activity. Thus, the additives i.e., 1% butanol was better enhancers of enzyme activity in the absence of Triton X-100 than in its presence (Table 1). However, 1% (V/V) ethanol, methanol, acetone and n-propanol improved the enzyme activity slightly more in the presence to Triton X-100 in the infiltration medium compared to – Triton X-100 levels.
Discussion
NR activity is the rate – limiting step in the process of nitrate reduction to ammonia 4. Several workers have recommended the use of intact tissue (in vivo) assay of NR 18,19. The in vivo assay described in this study is a simple and rapid way of assaying NR activity in the leaves of Punjab wheat.
The linearity in nitrite production by leaf segments equivalent to up to 400 mg in infiltration medium (Fig. 1) shows that the amount of NR did not vary with the amount of leaf tissue. This also facilitated us to use either 100 or 200 mg leaf tissue interchangeably depending on the nature of the tissue (greening shoots vs. green leaf tissue) 20.
The increase in in vivo NR activity with increase in external nitrate in the incubation medium (Fig. 2) indicates that the availability of the substrate nitrate to the enzyme was limiting and that 100mM external nitrate was required to saturate the enzyme. The lack of enzyme activity in the absence of external nitrate and the lower enzyme activities below 100 mM external nitrate in the infiltration medium shows that even though the leaf segments employed were harvested from seedlings induced with optimal nitrate, most of the endogenous nitrate was inaccessible to the reduction site possibly due to storage in the vacuole. Such a compartmentation of a large, inaccessible, storage pool (vacuolar) and a small, accessible, metabolic pool (cytoplasm) for nitrate was advanced 21 suggested the existence of barriers to the flow of nitrate from the storage pool to the reduction site but with relatively free access of exogenous nitrate. The requirement of 100mM external nitrate in the infiltration medium for optimal NR activity is in accordance with previous observations 20. Where 200mM external nitrite was optimum. This is probably because of the nature of the leaf tissue (a CAM plant) used by them and attendant barriers to flow of external nitrate because of increased leaf width and thickness compared to the leaf segments in the present investigation.
The requirement of external PH of 7.5 in the infiltration medium with almost equal decrease in activity in the acidic as well as alkaline PH (Fig. 3) differs from the more pronounced inhibition of enzyme activity by acidic pH reported by in a CAM plant 20. Though the PH optimum for NR activity has been reported to vary among plant species 22,23. The results on the effect of PH on the in vivo NR activity, when plotted, yielded a bell-shaped curve (Fig. 3), a characteristic of ideal, enzyme- catalyzed reactions influenced by PH. The acid liability of NADH and specifically NADPH has been taken as an index to suggest that the PH of the cytoplasm of plant cells is above 7.0 (approximately 7.2) 24. In as much as enzymes vary as to their PH optima, any change in cellular PH may result in considerable changes in the relative balances between metabolic pathways. The decrease in in vivo NR activity at PH below and above the optimum may conform to such a possibility, besides the pH- induced alterations in the binding of the cofactor or the substrate to the enzyme and liability of the enzyme and / or the cofactor or decreased saturation of the enzyme with the substrate because of decreased affinity or increased denaturation on both sides of the optimum. These effects may occur in combination.
The rate of an enzyme catalyzed reaction increases with rise in temperature till it reaches the maximum. The results in Fig. 4 corroborate this principle and show that the enzyme activity (velocity) decreased on further increasing the temperature. This effect of temperature may be due to several reasons including an effect on the stability of the enzyme, especially at elevated temperatures (Fig. 4). Though inhibitory effects of temperature on the activity of enzymes on either side of the optimum are most evident in enzyme assayed in vitro the similar effects observed in the present investigation on enzyme activity assayed in vivo even with intact leaf segments indicate that the enzyme nitrate reductase, though localized inside leaf cell cytoplasm, is as susceptible to temperature effects as that assayed in vitro. The stimulation of nitrite production by the various organic solvents in wheat leaf segments (Table 1) support the contention of the reports that monohydroxy alcholos stimulate nitrite production in leaf discs 21,25. This stimulation of nitrite production into the infiltration medium by organic solvents may stem either from increased nitrate reduction owing to the leakage of nitrate from the storage pool to the metabolic pool 21,26 or from increased membrane permeability for nitrate and nitrite 18,23. Other possible effects of the organic solvents on the in vivo NR activity may include: (1) reduction of surface tension with consequent improvement in contact of the tissue with the incubation (assay) medium 26 (2) decrease in the rate of diffusion of O2 into the leaf tissue as anaerobiosis or lowered O2 enhances nitrate reduction in vivo by inhibiting NADH oxidation by oxygen via the mitochondrial respiration 27. But, the superiority of 1% n-butanol to the rest of the monhydroxyalcohols is a new revelation and has not been reported so far. Hitherto, n-propanol has been reported to enhance nitrite production to the maximum in leaf segments of sorghum and other plant species including a CAM plant 20. The present findings (Table 1) for the first time report 1% n-butanol as the most effective monohydroxyalcohol. n-propanol has been reported to cause maximal nitrite production (54% over the control) by alleviating O2 inhibition of in vivo nitrate reduction 22.
Table 1:Effect Of Various Organic Solvents With And Without 0.1% Triton X-100 On In Vivo Nr Activity.
| NATURE OF THE MEDIUM
|
INCLUSIONS OF ORGANIC SOLVENT IN THE MEDIUM | µ MOLES OF
NO2 ¯ FORMED IN 1 Hr/ GRAM FRESH Wt. |
% OF ACTIVITY |
|
MEDIUM WITHOUT TRITON- X |
BUFFER+KNO3
(CONTROL-1) |
0.114 |
100 |
| CONTROL-1+1%METHANOL | 0.144 | 126.6 | |
| CONTROL-1+1%ETHANOL | 0.148 | 130 | |
| CONTROL-1+1%PROPANOL | 0.163 | 143 | |
| CONTROL-1+1%n-BUTANOL | 0.475 | 420 | |
| CONTROL-1+1%ACETONE | 0.125 | 110 | |
|
MEDIUM WITH TRITON-X |
BUFFER+KNO3+1%TRITON-X (CONTROL-2) |
0.430 |
100 |
| CONTROL-2+1%METHANOL | 0.607 | 141 | |
| CONTROL-2+1%ETHANOL | 0.657 | 153 | |
| CONTROL-2+1%PROPANOL | 0.670 | 156 | |
| CONTROL-2+1%n-BUTANOL | 0.942 | 219 | |
| CONTROL-2+1%ACETONE | 0.628 | 146 |
The 3.7- fold enhancement of enzyme activity by Triton X-100 over the control (table 1) concurs with reports of enhancement of NR activity 20 and might be due to Triton X-100 serving both as a surfactant and a detergent, thereby promoting membrane permeability. The decrease in the magnitude of promotion of nitrate reduction by the monohydroxyalocohols, 1% butanol in the presence of 0.1% Triton X-100 proves that presence of Triton X-100 and butanol does not lead to synergistic effect (Table 1). In fact, 1% n-butanol was better enhancers of enzyme activity in the absence of Triton X-100 than in its presence.
Acknowledgment
The authors acknowledge the support of the management and staff of NRC for Sorghum, MVR PG College and GITAM University, India for providing material and lab space in bringing out the above literature and experimentation.
References
- Keys A.J., The biological efficiency of protein production (Jones, J.G.W., ed.), Cambridge University press, 69-82 (1973).
- Schubert K.R., The energetics of biological nitrogen fixation workshop summaries – I, American Society of Plant Physiology, 30 (1982).
- Guerrero M.G., J. Rivas, A. Paneque and M. Losada, Mechanism of nitrate and nitrate reduction in Chlorella cells grown in the dark. Biochemical and Biophysical Research Communications, 45(1), 82-89 (1971).
- Beevers L. and R.H. Hageman, Nitrate Reduction in Higher Plants, Annual Review of Plant physiology, 20, 495 – 522 (1969).
- Beevers L. and R.H. Hageman, The role of light in nitrate metabolism in higher plants, Photophysiology (A.C. Giese, ed.), Vol. VII, Academic Press, New York, 85-113 (1972).
- Beevers L and R.H. Hageman, Nitrate and nitrite reduction, (Stumpf PK, Conn EE, eds.), The Biochemistry of Plants, Vol. V, Academic Press, New York 115-68. (1980).
- Hewitt E.J., Assimilatory nitrate-nitrite reduction, Annual Review of Plant Physiology, 26, 73-100 (1975).
- Hewitt E.J., D.P. Hucklesby and B.A. Notton, Nitrate metabolism, In: Plant Biochemistry (Bonner, J. and J.E.Varner, eds.), Academic press, New york., 633-681 (1976).
- Oaks A., Primary nitrogen assimilation in higher plants and its regulation, Canadian Journal of Botany, 72, 739-750 (1994).
- Warner R.L and Kleinhofs A., Genetics and molecular biology of nitrate metabolism in higher plants, Physiol. Plantarum, 85, 245-252 (1992).
- Campbell W.M., nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology, Annual Review of Plant Physiology and Plant Molecular Biology, 50, 277-303 (1999).
- Bloom AJ, Assimilation of Mineral Nutrition, In: Taiz L, Zeiger E (eds) Plant Physiology, 2nd Edition, Sinauer Assoc., Sunderland, MA, 323-345 (1998).
- Deckard E.L., R.J. Lambert and R.H. Hageman, Nitrate reductase activity in corn leaves as related to yields of grain and grain protein, Crop Science, 13, 343-350 (1973).
- Eilrich G.L. and R.H. Hageman, Nitrate reductase activity and its relationship to accumulation of vegetative and grain nitrogen in wheat (Triticum aestivum L.), Crop Science, 13, 59-66 (1973).
- Schrader L.E., D.A. Cataldo and D.M. Peterson, Use of Protein in Extraction and Stabilization of Nitrate Reductase, Plant Physiology, 53, 688-690 (1974).
- Wallace W. A, Nitrate Reductase Inactivating Enzyme from the Maize Root, Plant Physiology, 52, 197-201 (1973).
- Kumada H.J, The nitrate utilization in seed embryos of Vigna sesquipedalis, Journal of Biochemistry, 40, 439-451 (1953.).
- Jaworski E.G, Nitrate reductase essay in intact leaf tissues, Biochemical and Biophysical Research Communications, 43, 1274-1279 (1971).
- Harper J.E. and Hageman, R.H., Canopy and Seasonal Profiles of Nitrate Reductase in Soybeans (Glycine max L. Merr.), Plant Physiology, 49, 146-154 (1972).
- Balakumar T., M. Thangavel and Paliwal, Characteristics of in vivo Nitrate Reduction In The Cam Plant Notonia Grandiflora Dc., Photosynthetica, 28(2), 297-306 (1993).
- Ferrari T.E.., Yodev, O.C., and filner, P., Anaerobic Nitrite Production by Plant Cells and Tissues: Evidence for Two Nitrate Pools, Plant Physiology, 51, 423-431 (1973).
- Mann A.F., D.P. Hucklesby and E.J. Hewitt, Effect of aerobic and anaerobic conditions on the in vivo nitrate reductase assay in spinach leaves, Planta, 146, 83-89 (1979).
- Nicholas J.C., J.E. Harper, and R.H. Hageman, Nitrate reductase activity in soybeans (Glycine max [L.] Merr.). I. Effects of Light and Temperature, Plant Physiology, 58, 731-735 (1976).
- Davies D.D, Factors affecting protein turnover in plants. In EJ Hewitt, CV Cutting, eds, Nitrogen Assimilation in Plants, Academic Press, New York, 369-396 (1979).
- Lawrence J.M. and H.E. Herrick, Media for in vivo nitrate reductase assay of plant tissues, Plant Science, 24(1), 17-26 (1982).
- Jones R.W. and R.W. Sheard, Conditions affecting in vivo nitrate reductase activity in chlorophyllous tissues, Canadian Journal of Botany, 55, 896-901 (1977).
- Subbalakshmi B., S.P.Singh, S. Prakash and M.S.Naik, Regulation of nitrate reduction in wheat and rice leaves by oxygen and NADH supply, Plant Science, 14, 133-137 (1979).

This work is licensed under a Creative Commons Attribution 4.0 International License.





