How to Cite | Publication History | PlumX Article Matrix
M. V. V. Chandana Lakshmi1*, V. Sridevi1, Neeharika1, Beena1, M. Narasimha Rao2 and A. V. N. Swamy3
1Centre for Biotechnology, Department of Chemical Engineering, Andhra University, Visakhapatnam - 03 India.
2Al-Ameer College of Engineering and Information Technology, College of Engineering, Gudilova, Anadapuram, Visakhapatnam India.
3Department of Chemical Engineering, College of Engineering, J.N.T.U., Anantapur India.
Corresponding Author E-mail: mahantilakshmi@yahoo.com
ABSTRACT: Phenols are one of many commonly occurring organic pollutants in the environment. These compounds are stable and even at low concentrations they may be toxic towards living organisms and cause unfavorable chemical changes in waters and soils. Among others, biotechnological methods are applied for their removal. The biodegradation of phenol by Pseudomonas aeruginosa (NCIM 2074) and Pseudomonas desmolyticum (NCIM 2028), potential biodegradents have been investigated for its degrading potential under different operating conditions. It was found that the degrading potential of P. aeruginosa and P. desmolyticum was strongly affected by the variations in pH, inoculum size, temperature and carbon (glucose) source. Optimum conditions of the variables for the growth of P. aeruginosa and P. desmolyticum and for maximum biodegradation of phenol are pH (7, 6), inoculum size (5% v/v, 4% v/v), temperature (320C, 320C) and carbon (0.5 g/l, 0.5 g/l) respectively. These results are useful to understand the physiological and biochemical properties of P. aeruginosa and P. desmolyticum before its optimum use in environmental application and these data will assist in choosing the right phenol degrader for a changeable environment.
KEYWORDS: Biodegradation; pH; Inoculum size; Temperature; Carbon; Phenol; Pseudomonas aeruginosa
Download this article as:| Copy the following to cite this article: Lakshmi. M. V. V. C, Sridevi. V, Neeharika. Beena. Rao. M. N, Swamy. A. V. N. Comparative Study on Physico-Chemical Parameters on Biodegradation of Phenol by Pseudomonas Aeruginosa (NCIM 2074) and Pseudomonas Desmolyticum (NCIM 2028). Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Lakshmi. M. V. V. C, Sridevi. V, Neeharika. Beena. Rao. M. N, Swamy. A. V. N. Comparative Study on Physico-Chemical Parameters on Biodegradation of Phenol by Pseudomonas Aeruginosa (NCIM 2074) and Pseudomonas Desmolyticum (NCIM 2028). Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8846. |
Introduction
Phenol and its derivatives are the basic structural unit in a wide variety of synthetic organic compounds (Annadurai et al. 2000). It is an organic, aromatic compound that occurs naturally in the environment (Prpich and Daugulis, 2005), but is more commonly produced artificially from industrial activities such as petroleum processing, plastic manufacturing, resin production, pesticide production, steel manufacturing and the production of paints and varnish (Mahadevaswamy et al. 1997; Banyopadhyay et al. 1998). This aromatic compound is water soluble and highly mobile (Collins and Daugulis, 1997) and as such wastewaters generated from these industrial activities contain high concentrations of phenolic compounds (Change et al. 1998) which eventually may reach down to streams, rivers, lakes, and soil, which represent a serious ecological problem due to their wide spread use and occurrence throughout the environment (Fava et al. 1995).
Phenol is a listed priority pollutant by the U.S. Environmental Protection Agency (EPA, 1979) and is considered to be a toxic compound by the Agency for Toxic substances and Disease Registry (ATSDR, 2003). The adverse effects of phenol on health are well documented (Calabrese and Kenyon, 1991) and death among adults has been reported with ingestion of phenol ranging from 1 to 32 gm (Prpich and Daugulis, 2005). The low volatility of phenol and its affinity for water make oral consumption of contaminated water, the greatest risk to humans (Prpich and Daugulis, 2005).
A variety of techniques have been used for the removal of phenol from industrial effluents and contaminated waters with bioremediation receiving the most attention due to its environmentally friendly, its ability to completely mineralize toxic organic compounds and of low-cost (Kobayashi and Rittman, 1982; Prpich and Daugulis, 2005). Microbial degradation of phenol actively studied and these studies have been shown that phenol can be aerobically degraded by wide variety of fungi and bacteria cultures such as Candida tropicalis (Ruiz-ordaz et al. 2001; Chang et al. 1998; Ruiz-ordaz et al. 1998), Acinetobacter calcoaceticus (Paller et al. 1995), Alcaligenes eutrophus (Hughes et al. 1984; Leonard and Lindley, 1998), Pseudomonas putida (Hill and Robinson, 1975; Nikakhtari and Hill, 2006) and Burkholderia cepacia G4 (Folsom et al. 1990; Solomon et al. 1994). For high strength and low volumes of wastewaters, phenol removal by degradation technique using Pseudomonas sp., has been adopted (Annadurai et al. 1999).
The primary factor affecting the substrate decomposition rate in natural systems is pH. Degradation of phenol at various pH, plays a vital role in the growth, degradation and lysis of bacteria. (Annadurai et al.1999).
An effective bacterial inoculum should be able to tolerate high levels of phenol, while maintaining a level of activity to provide efficient mineralization (Shaw et al., 1997). Hence, inoculum size is a primary tool to achieve a good rate of phenol degradation.
Temperature exerts an important regulatory influence on the rate of metabolism (Ghosh and Swamynathan, 2003).
Besides, it has been suggested (Roszak and Colwell, 1987; Watanable et al. 1998) that other factor, such as the nutrient availability (carbon source), can also affect the bacterial growth.
In order to find a strain able to degrade phenol in a changeable environment, we studied the influence of the pH of the medium, the effect of inoculum size, effect of temperature and carbon source on phenol degradation by phenol degrading organisms – P. aeruginosa and P. desmolyticum and were compared.
Materials and Methods
Chemicals
Phenol (99% pure, chemical grade) 4-amino antipyrine and all other chemicals used were from Merck.
Source of organism
The microorganisms P. aeruginosa and P. desmolyticum were obtained from culture collection (NCL) Pune, India. The microorganisms were maintained on a medium containing Beef extract: 1.0 g/l, Yeast extract: 2.0 g/l, Peptone: 5.0 g/l, NaCl: 5.0 g/l and Agar: 20 g/l. The pH of the medium was adjusted to 7.0 by adding 1N NaOH. It was stored at 320C for further use.
Growth determination
To study the extent of degradation, the cells were grown in a Minimal Salts (MS) medium with the following composition: Phenol 0.500 g/l; K2HPO4, 1.5 g/l; KH2PO4, 0.5 g/l; (NH4)2SO4, 0.5 g/l; NaCl, 0.5 g/l; Na2SO4, 3.0 g/l; Yeast extract, 2.0 g/l; Ferrous sulfate, 0.002 g/l; CaCl2, 0.002 g/l in conical flasks containing and inoculated with P. aeruginosa and P.desmolyticum. The experimental studies were carried out in shake flasks with agitation at a rate of 120 rpm, temperature at 320C. Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Effect of pH of the medium on phenol degradation
Pseudomonas Cells were grown in MS medium with 500 mg/l of phenol at different pH values 5 to 9. This mixture was contained in 250 ml Erlenmeyer flasks. The cultures were placed on a shaker (120 rpm) at 320C. At different times, growth and phenol degradation were measured.
Effect of inoculum size on phenol degradation
The effect of inoculum size (1 – 10% v/v) on phenol degradation was tested. Cells were grown as shake cultures at 320C in MS medium supplemented with 500 mg/l phenol at pH 7 in case of P. aeruginosa and pH 6 for P.desmolyticum in 250 ml Erlenmeyer flasks. At different times, growth and phenol degradation were measured.
Effect of temperature of the medium on phenol degradation
aeruginosa and P.desmolyticum were grown in MS medium with 500 mg/l of phenol at different temperatures (300C, 320C, 330C and 340C) at pH 7, inoculum size 5% v/v and at pH 6, inoculum size 4% v/v respectively. This mixture was contained in 250 ml Erlenmeyer flasks individually. The cultures were placed on a shaker (120 rpm) at the above mentioned temperatures. At different times, growth and phenol degradation were measured.
Effect of carbon (glucose) source on phenol degradation
The effect of carbon (0.0, 0.5, 1, 2, 3 and 4 g/l) on phenol degradation of P. aeruginosa and P.desmolyticum was tested at pH 7, inoculum size 5% v/v, temperature 320C, and at pH 6, inoculum size 4% v/v, temperature 320C respectively. Cells were grown as shake cultures in MS medium supplemented with 500 mg/l phenol in 250 ml Erlenmeyer flask individually. At different times, growth and phenol degradation were measured.
Estimation of phenol
Phenol was determined quantitatively by the Spectrophotometric method (DR/ 4000 V, Hach) using 4-amino antipyrine as the color reagent (λ max: 500nm) according to standard methods of analysis (APHA, 1989).
Growth determination
Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Results and Discussion
Biological treatment using P. aeruginosa and P.desmolyticum was the effective method for removal of phenol. It is also a time saving method compared to other conventional methods.
Effect of pH of the medium on phenol degradation
pH values from 5 to 9 were investigated. Phenol was degraded rapidly at pH 7 by P. aeruginosa and at pH 6 by P.desmolyticum. In control cultures no aromatic ring cleavage occurred. At these pH values, phenol degradation was high compared to other pH values. The phenol concentration started decreasing after 24 h inoculation for both the microorganisms but the rate of degradation of phenol was high and faster by P. aeruginosa compared to P.desmolyticum as shown in (Fig.-1).
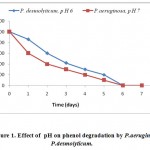 |
Figure 1: Effect of pH on phenol degradation by P.aeruginosa and P.desmolyticum. Click here to View figure |
The internal environment of all living cells is believed to be approximately neutral. Most organisms cannot tolerate pH levels below 4.0 or above 9.0. At low (4.0) or high (9.0) pH values, acid or bases can penetrate into cells more easily because they tend to exist in undissociated forms under these conditions and electrostatic force cannot prevent them from entering the cells. The permeated substances can upset the internal pH balance since the bacterial activity decreases as the pH deviates from neutral conditions.
Effect of inoculum size on phenol degradation
Phenol was degraded by P. aeruginosa and by P.desmolyticum during all the inoculum sizes (1-10% v/v) tested at pH 7 and at pH 6 respectively. At 5% v/v, P. aeruginosa and at 4% v/v, P.desmolyticum the phenol concentration began to decrease rapidly and complete degradation has taken place at 60 h for P. aeruginosa and at 70 h by P.desmolyticum. Cultures inoculated with 5 % v/v and 4% v/v inoculum sizes for P. aeruginosa and by P.desmolyticum showed the highest rate of phenol degradation as shown in Fig.-2, while the cultures inoculated with the other inoculum sizes showed a decrease in phenol consumption.
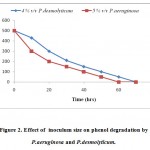 |
Figure 2: Effect of inoculum size on phenol degradation by P.aeruginosa and P.desmolyticum. Click here to View figure |
Effect of temperature of the medium on phenol degradation
Temperature exerts an important regulatory influence on the rate of metabolism. However, some work has been done on the microbiological activity of the organisms present in the water treatment plants operating at lower temperatures. But conventional biological waste treatment processes can operate at low temperature provided sufficient time is allowed for these organisms to degrade in organic wastes. Microbiological degradation of phenol in industrial wastewater is affected by temperature in an unexpected manner. The efficiency of treatment by microbiological activity on phenol and other contaminants were significantly good.
Temperature values (300C, 320C, 330C and 340C) were investigated for P. aeruginosa (pH 7, 5% v/v) and P. desmolyticum (pH 6, 4% v/v). Both the microorganisms degraded phenol rapidly at temperature 320C. At this temperature, the phenol concentration began to decrease rapidly after 24 h inoculation and degraded completely around 60 h by P. aeruginosa. Phenol was degraded rapidly by P. desmolyticum at 320C after 40hrs and complete degradation was found at around 70 h as shown in Fig.-3. Hence, P.aeruginosa had better degradation rate than P. desmolyticum.
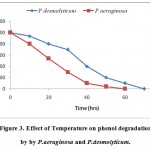 |
Figure 3: Effect of Temperature on phenol degradation by by P.aeruginosa and P.desmolyticum. Click here to View figure |
Effect of carbon (glucose) source on phenol degradation
The presence of glucose in the culture medium increased the tolerance of the organisms to high phenol concentrations by providing a good source readily metabolisable carbon to support cell growth. Hence, it was concluded that glucose on MS medium supported phenol degradation.
Phenol degradation by P. aeruginosa and P. desmolyticum at different concentrations of glucose (0.0, 0.5, 1.0, 2.0, 3.0, 4.0 g/l) was tested. Cultures inoculated with 0.5 g/l glucose for P.aeruginosa (pH 7, 5% v/v, 320C) and P. desmolyticum (pH 6, 4% v/v, 320C) showed the highest rate of phenol degradation as shown in Fig.-4, while the cultures inoculated with the other concentrations showed a decrease in phenol consumption.
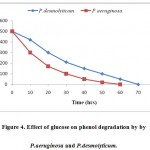 |
Figure 4: Effect of glucose on phenol degradation by by P.aeruginosa and P.desmolyticum. Click here to View figure |
In summary, these results shows P. aeruginosa is able to tolerate higher levels of phenol when supplemented with glucose as additional source compared to P. desmolyticum. Time is the limiting factor as the biodegradation of recalcitrant compounds can range from hours to years.
In Fig.-5 the variation of phenol concentration at their optimum conditions throughout both fermentations are shown. As can be seen, the removing rate of phenol by P.aeruginosa is slightly higher than that of P.desmolyticum.
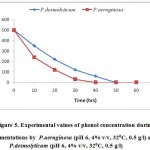 |
Figure 5: Experimental values of phenol concentration during fermentations by P.aeruginosa (pH 6, 4% v/v, 320C, 0.5 g/l) and P.desmolyticum (pH 6, 4% v/v, 320C, 0.5 g/l).
|
References
- Annadurai, G., Balan, S.M. and Murugesan, T. 2000. Design of experiments in the biodegradation of phenol using immobilized Pseudomonas pictorium (NCIM-2077) on activated carbon. Eng. 22: 101-107.
- Prpich, G.P. and Daugulis, A.J. 2005. Enhanced biodegradation of phenol by a microbial consortium in a solid-liquid two-phase partitioning bioreactor. 16: 329-339.
- Mahadevaswamy, M., Mall, I.D., Prasad, B. and Mishra, I.M. 1997. Removal of phenol by adsorption on coal fly ash and activated carbon. Res. 16(3): 170-175.
- Bandyopadhyay, K., Das, D. and Maiti, B.R.1998. Kinetics of phenol degradation using Pseudomonas putida MTCC 1194. Eng. 18: 373-377.
- Collins, L.D. and Daugulis, A.J.1997. Biodegradation of phenol at high initial concentration in two-phase partitioning batch and fed-batch bioreactors. Bioeng. 55: 155-162.
- Change, Y.H., Li, C.T., Chang, M.C. and. Shieh, W.K. 1998. Batch phenol degradation by Candida tropicalis and its fusant. Bioeng. 60: 391-395.
- Fava, F., Armenante, P.M., Kafkewitz, D. and Marchetti, L.1995. Influence of organic and inorganic growth supplements on the aerobic biodegradation of chlorobenzoic acid. Microbial. Biotechnol. 43:171-177.
- Calabrese, E.J. and Kenyon, E.M. Air toxics and Risk Assessment. Lewis publishers, Chelsea. MI. 1991.
- Kobayashi, H. and Rittman, B.E. 1982. Microbial removal of hazardous organic compounds. Sci. Technol. 16:170-183.
- Ruiz-ordaz, N., Ruiz-Lagunez, J.C., Castanou-Gonzalez, J.H., Hernandez-Manzano, E., Cristiani-Urbina, E. and Galindez-Mayer, J. 2001. Phenol biodegradation using a repeated batch culture of Candida tropicalis in a multistage bubble column. Revista Latinoamericana de Microbiogia. 43: 19-25.
- Ruiz-ordaz, N., Ruiz-Lagunez, J.C., Castanou-Gonzalez, J.H., Hernandez-Manzano, E., Cristiani-Urbina, E. and Galindez-Mayer, J. 1998. Growth kinetic model that describes the inhibitory and lytic effects of phenol on Candida tropicalis yeast. Biotechnol. Prog. 14: 966-969.
- Paller, G., Hommel, R.K and Kleber, H.P.1995. Phenol degradation by Acinetobacter calcoaceticus NCIB 8250. J. Basic Microbial.35: 325-335.
- Hughes, E.J., Bayly, R.C. and Skurray, R.A. 1984. Evidence for isofunctional enzymes in the degradation of phenol , m- and p- toluate, and p- cresol via catechol metacleavage pathways in Alcaligenes eutrophus. Bacteriol. 158: 79-83.
- Leonard, D. and Lindley, N.D. 1998. Carbon and energy flux constraints in continuous cultures of Alcaligenes eutrophus grown on phenol. Microbiology. 144: 241-248.
- Hill, G.A. and Robinson, C.W. 1975. Substrate inhibition kinetics: Phenol degradation by Pseudomonas putida. Biotechnol. Bioeng. 17: 599-615.
- Nikakhtari, H. and Hill , G.A. 2006. Continuation bioremediation of phenol- polluted air in an external loop airlift bioreactor with a packed bed. Chem. Tech. Biotechnol. 81(6): 1029-1038.
- Folsom, B.R., Chapman, P. J. and Pritchard, P.H. 1990. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: Kinetics and interactions between substrates. Appl. Environ. Microbial. 57: 1279-1285.
- Solomon, B.O., Posten, C., Harder, M.P.F., Hecht, V. and Deckwer, W-D. 1994. Energetics of Pseudomonas cepacia growth in a chemostat phenol limitation. Chem. Technol. Biotechnol. 60: 275-282.
- Annadurai, G., Mathalai Balan, S. and Murugesan, T. 1999. Box- Behnken design in the development of optimized complex medium for phenol degradation using Pseudomonas putida (NCIM 2174). Eng. 21: 415-421.
- Shaw, K.W., Lee, H. and Trevors, J. 1997. Effect of initial cell density, substrate concentration and temperature on penta chloro phenol degradation by Pseudomonas , UG-30. J. Chem. Technol. Biotechnol. 69:107-113.
- Ghosh, S. and Swaminathan, T. 2003. Optimization of process variables for the extractive fermentation of 2,3 – butanedion by Klebsiella oxytoca in aqueous two- phase system using response surface methodology. Biochem. Eng. Q 17(4): 319-325.
- Roszak, D.B. and Colwell, R.R .1987. Survival strategy of bacteria in the natural environment. Rev. 51: 207-214.
- Watanabe, K., Yamamoto, S., Hino, S. and Harayama, S. 1998. Population dynamics if phenol-degrading bacteria in activated sludge determination by gyrB-targeted quantitative PCR. Appl. Environ. Microbiol. 64: 1203-1209.
- American Public Health Association (APHA), American Water Works Association, Water Pollution Control Federation. Standard methods for the examination of water and wastewater. 17th edition. Washington, D.C. American Public Health Association.1989. 9-55 – 9-62.

This work is licensed under a Creative Commons Attribution 4.0 International License.





