How to Cite | Publication History | PlumX Article Matrix
Effect of Ph and Inoculum Size on Phenol Degradation by Pseudomonas Desmolyticum (NCIM 2028)
V. Sridevi* and M. V. V. Chandana Lakshmi
Department of Chemical Engineering (Biotechnology), Andhra University, Visakhapatnam - 530 003 India.
Corresponding Auhtor E-mail: vellurusridevi@yahoo.com
ABSTRACT: Phenolic compounds are hazardous pollutants that are toxic relatively at low concentrations. Accumulation of phenol creates toxicity both for flora and fauna. Because of its toxicity, there is a need to decontaminate the phenol-laden soils hence, bioremediation is a very useful alternative to conventional clean-up methods. The aim of this work was to study the effect of inoculum size and the influence of pH on phenol degradation by Pseudomonas desmolyticum. Phenol was degraded rapidly at pH 5 to 9, but the maximum rate of phenol degradation by P. desmolyticum was at pH 6. In contrast, the phenol degradation at pH (pH 5, 7, 8 and 9) were significantly lower, although phenol was totally depleted. Phenol was degraded at every inoculum size tested (1 – 10% v/v) but the maximum rate of phenol degradation was observed at 4% v/v in batch experimental system.These results are useful to understand the physiological and biochemical properties of P. desmolyticum before its optimum use in environmental application and these data will assist in choosing the right phenol degrader for a changeable environment.
KEYWORDS: Biodegradation; inoculum size; pH; phenol; Pseudomonas desmolyticum
Download this article as:| Copy the following to cite this article: Sridevi. V, Lakshmi. M. V. V. C. Effect of Ph and Inoculum Size on Phenol Degradation by Pseudomonas Desmolyticum (NCIM 2028). Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Sridevi. V, Lakshmi. M. V. V. C. Effect of Ph and Inoculum Size on Phenol Degradation by Pseudomonas Desmolyticum (NCIM 2028). Biosci Biotechnol Res Asia 2009;6(2) . Available from: https://www.biotech-asia.org/?p=8984. |
Introduction
The massive increase in the synthesis of organic chemicals by man has led to the production of wide variety of compounds, some of which are xenobiotic. Their xenobiotic character means that their structures are not easily recognized by existing degradative enzymes and as a result they accumulate in the environment (Singleton, 1994). As they persist in the environment, they are capable of long-range transportation, bioaccumulation in human and animal tissue and biomagnifications in food chain. Phenol and its higher homology are aromatic molecules containing hydroxyl group attached to the benzene ring structure. The origin of phenol in the environment is both natural and industrial. Natural sources of phenol include forest fire, natural run off from urban area where asphalt is used as the binding material and natural decay of lignocellulosic material. Industrial sources such as oil refineries, chemical, petrochemical, pharmaceutical, metallurgical, pesticide products, paint and varnish industries, textile and also in the polymer industries like phenolic resins, bisphenol A, alkylphenols, caprolactums and adipic acid (Paula et al. 1998). The presence of phenol in water imparts carbolic odor to receiving water bodies and can cause toxic effects on aquatic flora and fauna (Ghadhi and Sangodkar, 1995). It is lethal to fish even at relatively low concentrations of 5- 25 mg/l (Saha et al. 1999). Phenols are toxic to human beings and effects several biochemical functions (Nuhoglu and Yakin, 2005). The concentration of phenols in waste waters varies from 10 to 300 mg/l. Phenol is also a priority pollutant and is included in the list of EPA (1979) (Indu Nair et al. 2008). As a result, phenol – containing effluents have to be properly treated prior to discharge (Keith, 1976; Jungclaus eta l. 1978; Parkhurst et al. 1979; Pfeffer, 1979; Delfino and Dube, 1976). Efficient treatment methods are necessary to reduce phenol concentration in waste water to acceptable level, which is 5 ppm (USEPA).
Conventional methods of treatment for phenolic wastes have been largely chemical or physical methods like chlorination, advanced oxidation process (Santigo et al. 2002), adsorption, solvent extraction, coagulation, flocculation, reverse osmosis, ozonation, photo catalysis and electrolytic oxidation (Arutchelvan et al. 2006), but these process have led to secondary effluent problems. Biological treatment for the bulk removal of these pollutants is therefore generally preferred. Biological degradation of phenol has been extensively studied using pure and mixed cultures (Kang and Park, 1997; Hugues, 1996; Wang et al. 1996; Ha et al. 2000; Chirwa and Wang, 2002). Few studies have been carried out with the bacterium P. desmolyticum in pure cultures (Kalme et al. 2007) in which phenol is degraded via the meta-pathway (Sala-Trepat et al. 1972). The success of bioremediation may depend on the availability of microbial strains that can mineralize high levels of phenol and withstand adverse conditions to complete under in situ conditions. An effective bacterial inoculum should be able to tolerate high levels of phenol while maintaining a high level of activity to provide efficient mineralization (Shaw et al. 1997). Understanding the physiological and biochemical properties of phenol degradation bacteria is required before optimum use of bacteria in environmental applications.
The biodegradation of phenol by P. desmolyticum (NCIM 2028), a potential biodegradent of phenol has been investigated for its degradation potential under different operating conditions. Two variables of pH and inoculum size were used to identify the significant effects and interactions in the batch studied.
Materials And Methods:
Chemicals
Phenol (99% pure, chemical grade) 4-amino antipyrine and all other chemicals used were from Merck.
Source of organism
The microorganism P. desmolyticum (NCIM 2028) was obtained from culture collection (NCL) Pune, India. The microorganism was maintained on a medium containing Beef extract: 1.0 g/l, Yeast extract: 2.0 g/l, Peptone: 5.0 g/l, NaCl: 5.0 g/l and Agar: 20 g/l. The pH of the medium was adjusted to 7.0 by adding 1 N NaOH. It was stored at 32oC for further use.
Growth determination
To study the extent of degradation, the cell were grown in a minimal salts (MS) medium with the following composition: Phenol 0.500 g/l; K2HPO4, 1.5 g/l; KH2PO4, 0.5 g/l; (NH4)2SO4, 0.5 g/l; NaCl, 0.5 g/l; Na2SO4, 3.0 g/l; yeast extract, 2.0 g/l; Ferrous sulfate, 0.002 g/l; CaCl2, 0.002 g/l in conical flask containing and inoculated with P. desmolyticum (NCIM 2028). The experimental studies were carried out in shake flask with agitation at a rate of 120 rpm and temperature 32oC. Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500 nm.
Influence of pH of the medium on phenol degradation
Pseudomonas Cells were grown in MS medium with 500 mg/l of phenol at different pH (5 to 9). The experiments were carried out in conical flasks containing MS medium and was inoculated with P. desmolyticum (NCIM 2028) and kept at 120 rpm, 32oC. At different times, growth and phenol degradation were measured.
Effect of inoculum size on phenol degradation
The effect of inoculum size (1 -10% v/v) on phenol degradation was tested. Cells were grown as shake cultures at 120 rpm, 32oC in MS medium supplemented with 500 mg/. phenol at pH 6 in 250 ml Erlenmeyer flask. At different times, growth and phenol degradation were measured.
Estimation of phenol
Phenol was determined quantitatively by the Spectrophotometric method (DR / 4000 V, Hach) suing 4-amino antipyrine as the color reagent (λmax: 500nm) according to standard methods of analysis (APHA, 1989).
Growth determination
Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Results and Discussion
Biological treatment using P. desmolyticum (NCIM 2028) was the most effective method for removal of phenol. It is also a time saving method compared to other conventional methods.
Influence of pH of the medium on phenol degradation
Five pH values from 5 to 9 were investigation in (Fig. -1). Phenol was degraded rapidly at pH 6. At this pH value, phenol degradation was high compared to other pH values. However, the phenol degradation at pH 5, 7, 8 and 9 was slower and phenol concentration decreased rapidly after 24 h inoculation. These results showed that P. desmolyticum degraded move phenol per day at pH 6 than at other pH value.
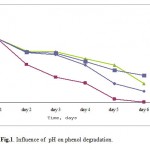 |
Figure 1: Influence of pH on phenol degradation.
|
Effect of inoculum size on phenol degradation
Phenol was degraded by P. desmolyticum during all the inoculum sizes (1-10% v/v) tested in (Fig. – 2). At 4% v/v the phenol concentration began to decrease rapidly after 5 h and reached 5 mg/l after approximately 70 h. However, in cultures receiving lower inoculum densities there was a progressive decrease of phenol concentration.
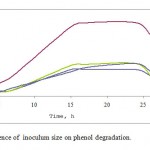 |
Figure 2: Influence of inoculum size on phenol degradation.
|
In addition, the rate of phenol degradation was tested. Cultures inoculated with 4% v/v inoculum size showed the highest rate of phenol degradation, while the cultures inoculated with the other inoculum size tested, showed a decrease in phenol consumption.
References
- Singleton, I. Microbial metabolism of xenobiotics: Fundamental and applied research. Chem. Biotechnol. Vol. 59: 9-23 (1994).
- Paula, M., Van Schei. and Young, L. Y. Isolation and characterization of phenol degradation denitrifying bacteria, Environ. Mcrobiol., Vol. 64: 2432-2438 (1998).
- Ghadhi, S. C. and Sangodkar, U. M. X. Potentials of Pseudomonas cepacia PAA in bioremediation of aquatic wastes containing phenol, Cochin. Proceedings of National Symposium Frontiers in applied and Environmental Microbiology, Dec. 11-13 (1995).
- Saha, N. C. Bhunia, F. and Kaviraj, A. Toxicity of phenol to fish and aquatic ecosystem, Bull Environ. Contam. Toxical., Vol 63: 195-202 (1999).
- Nuhoglu, A. and Yakin, B. Modeling of phenol removal in a batch reactor, Biochem. Vol. 40: 233-239 (2005).
- Indu Nair, C., Jayachandran, K. and Shankar Shashidhar, Biodegradation of phenol, African J. Biotechnol., 7: 4951-4958 (2008).
- Keith, L. H. Identification of organic compounds in unbleached treated kraft paper mill wastewaters, Sci. Technol., Vol 10: 555-564 (1976).
- Jungclaus, G. A., Lopez-Avila, V. and Hites, R. A. Organic compounds in an industrial wastewater. A case study of their environment impact, Sci. Technol., Vol. 12: 88-96 (1978).
- Parkhurst, B. R., Bradshaw, A. S. and Forte, J. L., An evaluation of the acute toxicity to aquatic biota of a coal conversion effluent and its major compounds, Environ. Contam. Toxicol., Vol. 23: 349-356 (1979).
- Pfeffer, F. M. The 1977 screening survey for measurements for measurement of organic priority pollutants in petroleum refinery waste waters, ASTM Spec. Tech. Publ., 181-190 (1979).
- Delfino, J. and Dube, D. J. Persistent contamination of ground water by phenol, Sci. Health, Vol. A11: 345-355 (1976).
- Santiago Esplugas, Jaime Glimenez, Sandra Contreras, Esther Pascual, Miguel Rodriguez Comparison of different advance oxidation processes for phenol degradation, Water Res., 36: 1034-1042 (2002).
- Arutchelvan, V., Kanakasabi, V., Elagovan, R., Nagarajan, S. and Muralikrishna, V. Kinetics of high strength phenol degradation using Bacillus brevis. J. hazardous Mat., 129: 216-222 (2006).
- Kang, M. H. and Park, J. M. Sequential degradation of phenol and cyanide by a commensal interaction between two microorganisms, Technol. Biotechnol, Vo. 69: 226-230 (1997).
- Hugues, S. M. and Cooper, D. G. Biodegradation of Phenol using the self-cycling fermentation (SCF) process, Bio. Engg., Vol. 51: 112-119 (1996).
- Wang, K. W. Baltzis, B. C. and Lewandowski. Kinetics of phenol biodegradation in the presence of glucose, Bio. Engg., Vol. 51: 87-94 (1996).
- Ha, S. R., Vinitnantharat, S. and Ozqki. H. Biodegradation by mixed microorganisms of granular activated carbon loaded with a mixture of phenols. Lett., Vol. 22: 1093-1096 (2000).
- Chirwa, E. N. and Wang, Y. T. Simultaneous Chromium (VI) reduction and phenol degradation in an anaerobic consortium of bacteria, Res., Vol. 34: 2376-2384 (2002).
- Kalme, S. D., Parshetti, Jadhar, S. U. and Govindwar, S. P. Biodegradation of benzidine based dye Direct Blue-6 by Pseudo Smonas desmolyticum NCIM 2112. Bioresource Technology, 98: 1405-1410 (2007).
- Sala-Trepat, J. M., Murray, J. M. and William, P. A. The metabolic divergence in the meta-cleavage of catechols by Pseudomonas putida NCIB 10015 Physiological significance and evolutionary implications. Biochem., Vol. 28: 347-456 (1972).
- Shaw, K. W. , Lee, H. and Trevors, J. Effect of initial cell density, substrate concentration and temperature on pentachlorophenol degradation by Pseudomonas sp. UG-30. Chem. Technol. Biotechnol., Vol. 69: 107-113 (1997).
- American Public Health Association (APHA), American Water Works Association, Water Pollution Control Federation. Standard methods for the examination of water and wastewater. 17th Washington, D.C. American Public Health Association. 9-55 – 9-62 (1989).

This work is licensed under a Creative Commons Attribution 4.0 International License.





