How to Cite | Publication History | PlumX Article Matrix
Formulation and Evaluation of Diclofenac Sodium Delayed Release Tablets
V. RavichandIran
Department of Pharmaceutics,Vels college of Pharmacy, Pallavaram, Chennai India.
Corresponding Author E-mail: sen03mpharm@gmail.com
ABSTRACT: Delayed release tablets of Diclofenac sodium tablets were prepared by wet granulation method using different pH resistant polymers like HPMC, CAP and Eudragit to dissolve it in alkaline pH. F1-F4 trials were performed and evaluated. F4 was found to be satisfactory. F4 was seal coated using HPMC E-5 EP & PEG 400L(F5). F5 was enteric coated using HPMC (F6-F7), CAP(F8-F9), EUDRAGIT (F10-F11).Dissolution was carried out in 0.1 N HCl for first 2hrs and next 1hr in phosphate buffer pH6.8. Dissolution studies of EUDRAGIT polymer trail (F10) shows much better release then HPMC and CAP.
KEYWORDS: Diclofenac sodium; Enteric coated tablets; Delayed release tablets; pH resistant polymers
Download this article as:| Copy the following to cite this article: RavichandIran. V.Formulation and Evaluation of Diclofenac Sodium Delayed Release Tablets. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: RavichandIran. V.Formulation and Evaluation of Diclofenac Sodium Delayed Release Tablets. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8869. |
Introduction
Diclofenac sodium is a non- steroidal anti inflammatory agent1, which is widely used in the long term therapy for rheumatoid arthritis. The biological half life of diclofenac sodium is about 1-2h, therefore, it requires multiple dosing to maintain therapeutic drug blood level. The most frequent adverse side effect of Diclofenac sodium on long term administration are GI disturbences8,peptic ulceration & gastro intestinal bleeding .Diclofenac sodium is poorly soluble in water & acidic pH (1-3) but rapidly soluble in alkaline pH(5-8)hence an attempt was made to formulate a delayed release2 tablet i.e. enteric coating3 of Diclofenac sodium which will released in alkaline pH(5-8) which eliminates GI disturbances 4& provides better patient compliance.
Materials and Methods
Diclofenac sodium delayed release tablets (F1-F4) were prepared by wet granulation method by using Lactose monohydrate7, Maize starch, Povidone, Micro crystalline cellulose, Colloidal silicone dioxide, Magnesium stearate11, PEG 400, HPMC E5 EP with various ratios of CAP,HPMC,& EUDRAGIT L30D55. Preformulation studies of pure drug and mixed blend were carried out for organoleptic properties, Angle of repose ,Bulk density, Tapped density, Carr’s index & Hausner’s ratio. After compression, the tablets were evaluated for Weight variation, Thickness, Hardness, Friability, drug content uniformity & invitro dissolution studies as per standards.
Preparation of Core Tablets (F1-F4)
Core tablets were prepared by wet granulation method9 by using PVPK 30 binder solution10.Which is mixed with diclofenac sodium, lactose monohydrate, corn starch were shifted through sieve no 40 and mixed for 10 min. Granules were dried and achieved LOD < 2.0%. Dried granules were passed through sieve no 18 .Micro crystalline cellulose PH102 & corn starch 400L which is passed through sieve no 40 should be added to above mixture. Megnesium sterate which is passed through sieve no 60 was to added to above mixture and compression was carried out by using 7mm punches. Results are shown in table 1
Preparation of Seal Coated Tablets F5
HPMC E5 was weighed & added water slowly under stirring. For this PEG 400L was added under stirring till clear solution obtained. Among F1-F4 core tablets one batch is optimized and taken for seal coating at suitable temperature &rpm.
Preparation of Enteric Coated Tablets
Seal coated tablets were taken and coated with different enteric coated solutions using HPMC,CAP&EUDRAGIT.table1.
Table1.Formulation of enteric coated tablets
| INGREDIENTS | F 6 | F 7 | F 8 | F 9 | F 10 | F 11 |
| CORETABLET(mg/tab) | ||||||
| Diclofenac sodium | 50 | 50 | 50 | 50 | 50 | 50 |
| Lactose monohydrate | 60 | 60 | 60 | 60 | 60 | 60 |
| Starch | 56 | 56 | 56 | 56 | 56 | 56 |
| Povidone | 4 | 4 | 4 | 4 | 4 | 4 |
| Sodium starch glycolate | 4 | 4 | 4 | 4 | 4 | 4 |
| Water | q.s* | q.s* | q.s* | q.s* | q.s* | q.s* |
| Microcrystalline cellulose | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 |
| Silicon dioxide | 5 | 5 | 5 | 5 | 5 | 5 |
| Magnesium stearate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| SEAL COAT(mg/tab) | ||||||
| HPMC E-5 EP | 3.28 | 3.28 | 3.28 | 3.28 | 3.28 | 3.28 |
| PEG 400 L | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 |
| ENTERIC COAT (mg/tab) | ||||||
| Percentage of coating | 8% | 11% | 8% | 11% | 8% | 11% |
| Cellulose acetate phthalate | 13.400 | 18.425 | — | — | — | — |
| HPMCP | — | — | 14.77 | 20.308 | – – – | – – – |
| Eudragit L-30 D55@ | – – – | — | — | – – – | 14.00 | 19.25 |
| Talc | 0.520 | 0.715 | – — | – – – | 2.320 | 3.19 |
| Diethyl phthalate | 2.400 | 3.300 | 1.55 | 2.132 | — – | – – – |
| Isopropyl Alcohol | – – – | – – – | – – – | – – – | q.s.* | q.s.* |
| Acetone | – – – | – – – | – – – | — – | q.s.* | q.s.* |
| Water | q.s.* | q.s.* | q.s.* | q.s.* | — – | — – |
Table 2 :physical parameters of blend.
| Batch No. | Angle
Angle of repose |
Bulk bulk
Density (gm/ml) |
Tapped tapped
Density (gm/ml) |
%
%Compr-essibility |
Hausner
Hausner’sRatio |
| F1 | 33.29 | 0.7620 | 0.8630 | 14.1 | 1.188 |
| F2 | 31.30 | 0.7711 | 0.8723 | 13.9 | 1.178 |
| F3 | 29.21 | 0.7810 | 0.8920 | 12.9 | 1.1499 |
| F4 | 26.45 | 0.7812 | 0.8928 | 12.5 | 1.1412 |
Charcterization of Blends
Prior to compression blends were evaluated for their characteristic parameters.
The results were shown in table. 3
Table 3
| Concentration(µg/ml) | Absorbance (nm) |
| 0 | 0 |
| 5 | 0.153 |
| 10 | 0.301 |
| 15 | 0.483 |
| 20 | 0.643 |
| 25 | 0.812 |
| 30 | 0.97 |
Characterization of tablets
The properties of coated tablets, such as thickness, weight variation, hardness, friability, disintegration time was determined using standard procedures .Briefly hardness was determined by using Monsanto hardness tester, friability was determined using Roche friabilator apparatus. The drug content was determined as described for blend. The results were shown in following table.
Evaluation of tablets 12
Physical parameters of formulated tablets like Weight variation, Hardness, Thickness, Frianility.& % of coating are shown in tables 4 &5.
Table 4: evaluation of core tablets and seal coated tablets.
| BATCH
No. |
Weight
(mg) |
Thickness
(mm) |
Hardness
(kg/cm2) |
Friability
(%) |
D.T.
(Min) |
|
F1 |
199.5 |
3.70 |
4.2 |
0.23 |
15 |
|
F2 |
200 |
3.75 |
4.1 |
0.32 |
12 |
|
F3 |
201.5 |
3.75 |
3.9 |
0.38 |
10 |
|
F4 |
200 |
3.77 |
3.8 |
0.29 |
8 |
| Seal coated tablets F5 | |||||
| F5 | 204 | 3.78 | 8.0 | 0.32 | 10 |
Table 5: Evaluation of Enteric coated tablets.
| BATCH NO. | Weight (mg) | Thickness (mm) | Hardness in kg/cm2 | Friability | %Coating |
| F6 | 220.5 | 3.99 | 8.32 | 0.24 | 8% |
| F7 | 224.2 | 4.00 | 8.12 | 0.32 | 11% |
| F8 | 220.9 | 3.98 | 8.31 | 0.29 | 8% |
| F9 | 224.6 | 4.01 | 8.01 | 0.33 | 11% |
| F10 | 221.1 | 3.99 | 8.12 | 0.35 | 8% |
| F11 | 224.9
|
4.00 | 8.23 | 0.28 | 11% |
Invitro Dissolution Studies13-14
The invitro dissolution studies were performed using USP II dissolution apparatus15 paddle type at 50 rpm. The dissolution studies were carried out for 2hrs in 0.1 N Hcl and the subsequently with Phosphate buffer pH 6.8 for next 1 hr at 37°c. the release studies were conducted in triplicate . Aliquots of sample (5ml) were with drawn at specific time intervals and drug content was determined at spectrophotometrically at 278 nm .the invitro release studies data obtained was treated to zero order equation,first order equation.Higuchi equation(Q=Kt1/2) and Korseymer peppas to know the drug release mechanism from enteric coated tablet.showin in figures 3,4.5&6
Results and Discusssion
The blend for tablets were prepared and characterized with respect to angle of repose ,bulk density,tapped density,carr’s index.the other parameters for blend were also determined and found to be in acceptable range.
The tablets of different formulations(F1-F10) were subjected to various evaluation tests such as weight variation ,friability,hardness& content uniformity according to procedure specified in IP.the weight variation and friability was less than 200.1mg & 0.29% for F1-F4,204.4 mg &0.32% for F5,222.7mg&0.35% for F6-F11.drug content uniformity was more than 955 in all batches in vitro release profile different concentrations of HPMC(8%&11%)F6 &F7,CAP(8%&11%)F8&F9 &EUDRAGIT(8%&11%)F10& F11.the tablets with the tablets with 8% optimum concentration shows significant release at 2 hr to 3hr. The EUDRAGIT polymer showed much better release than the other two polymers used for thw same purpose where as with the same weight given as that of HPMC,CAP& EUDRAGIT coated tablets showed release in 0.1 N Hcl. The % of coating is increase upto 11% in CAP & HPMC.there is no release in 0.1 N Hcl. Among the enteric coated tablets F 10 was the best.
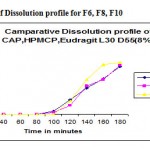 |
Figure 1: Comparitive of Dissolution profile for F6, F8, F10.
|
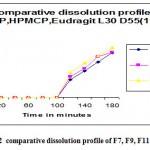 |
Figure 2: comparative dissolution profile of F7, F9, F11.
|
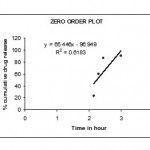 |
Figure 3
|
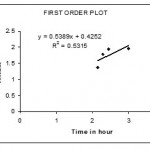 |
Figure 4
|
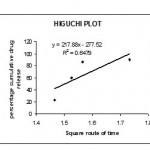 |
Figure 5
|
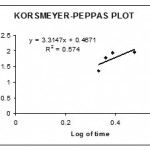 |
Figure 6
|
Conclusion
Result of present study demonstrated that pH resistant polymers could be employed for successfully for formulated enteric coated tablets of Diclofenac sodium.. The investigated delayed release tablet was capble of release drug in alkaline pH but in acid pH. This can be expected to avoid the GI disturbance and reduces dose of administration.
References
- Wan LS,chul WK. Deviation of the ratio of drugs in a two component mixture encapsulated in cellulose phthalate enteric coated tablets .J Microencapsul 1995;12:417-23
- J.Rathbone,MS Rathbone, Modified – Release drug delivery technology, volume-1,239-250
- JVV MCGINITY Aqeous polymeric coating for pharmaceutical dosage forms – 1997,pg no 81-95,227-267
- GA Agyilirah, GS Banker, A Shellac ,Polymers for controled Drug Delivery -1991,179-233 Chowhan Z.; Pharmaceutical Technology: Excipients and their functionality in drug product development; 245-286
- Raymond c, Amazon C et al ., hand book of pharmaceutical exciepient ,4th edition ,225-255, 195-199 ,433-439 ,305-308.
- Raymond c rowe ed. Hand book of pharmaceutical excipients. 4th edition publisher’s pharmaceutical press and American pharmaceutical association 450-482
- Chowhan Z.; Pharmaceutical Technology: Excipients and their functionality in drug product development; 245-286
- Pharmacological basis of therapeutics”. Goodman A, Gilman LS, Goodman TW, Rall F, eds. McMillan. 7th Edition, New York.
- Jorgensen A., Luukkonen P., Rantaren J., Schefer T., Juppo A. And Yllruusi J.; Jouranl of Pharmaceutical Sciences: Comparison of torque measurements and near-infrared spectroscopy in characterization of a wet granulation process; 2004; 93(9): 2232-2243.
- Ferrari F., Bertoni M., Bonferoni M. and Rossi S.; International Journal of Pharmaceutics: Investigation on bonding and disintegration properties of pharmaceutical materials; 1996; 13: 71-79.
- Guyot-Hermann; S.T.P. Pharmaceutical Science: The disintegration and disintegrating agent; 1992; 2(6): 445-462.
- M Rani, B Mishra Comparative; Evaluation of in vitro performance of commercial and fabricated sustained release diclofenac sodium tablets. Indian J Pharm SciYear : 2001,|Volume : 63 , Issue : 3, Page : 247-250
- E. A. Hosny ,G. M. El-Mahrouk ,M. W. Gouda; Formulation and in Vitro and in Vivo Availability of Diclofenac Sodium Enteric-Coated Beads drug development and industrial pharmacy, Volume 24, Issue7,July 1998 ,
- Qi XL,Zhu JB,Chen SJ ; Preparation and invitro evolution of diclofenac sodium microspeares using Eurdagit L30 D55 polymer International journal of pharmaceutical science, 2005,512-516
- Biju SS,Saisivam S,Rajan NS,Mishra PR studied the dissolution process of sodium diclofenac granules coated with a polymeric suspension of Eudragit L-30D-55. indian journal of pharmaceutical science 2008, vol 73,issue 1,page;43-49

This work is licensed under a Creative Commons Attribution 4.0 International License.





