How to Cite | Publication History | PlumX Article Matrix
S. Revathy1*, K. S. Sridharan2, A. S. Elumalai 3 And Umasekar3
1Department of Biochemistry and Biotechnology, Annamalai University, Chidhambaram (India).
2Department of Microbiology, Sri Ramachandra University, Chennai - 116 (India).
3Department of Plant Biology and Biotechnology, Presidency College, Chennai - 005 (India).
ABSTRACT: Enterococcus is one of the important causes of hospital acquired infections. Drug resistance particularly for High Level Aminoglycosides Resistance is one of the important patterns of resistance exhibited by Enterococcus. This study was conducted to determine the high-level aminoglycoside resistance for gentamicin and streptomycin in Enterococcal species isolated from a tertiary care centre. Number of isolates collected were 76 of which 42 (55.2%) from urine 26(34.21%) from exudates, 4(5.26%) from blood and rest from other body fluids. Most of the isolates were Enterococcus faecalis (72%) followed by Enterococcus faecium (28%). All the isolates were subjected to Kirby-Bauer disk diffusion test using high-content gentamicin (120 μg/disk) and streptomycin (300 μg/disk), single concentration agar dilution test with streptomycin 2000 μg/ml, 500 μg/ml and 2000 μg/ml of gentamicin. There were 27 (35.5%) were showed High Level Aminoglycosides Resistance (HLAR), of which 18(23.68%) were High Level Gentamycin Resistance (HLGR), 9(11.84%) were High Level Streptomycin Resistance (HLSR) and 9 (11.84%) isolates showed resistant to both. E.faecalis was the species most frequently exhibited High Level Resistance (HLR) for the drugs tested. Prevalence of HLAR in Enterococci is high and could be a serious problem in a hospital setup, screening for high-level aminoglycoside resistance must be included in routine antibiotic susceptibility reporting for Enterococcal isolates, especially from blood as high-level resistant strains does not show synergism with cell wall acting antibiotics like penicillin group and vancomycin which are the drug of choice.
KEYWORDS: High-Level Aminoglycoside Resistance (HLAR); Disk Diffusion test
Download this article as:| Copy the following to cite this article: Revathy. S, Sridharan. K. S, Elumala. A. S, Umasekar. Phenotypic Detection of High-Level Aminoglycoside Resistance (HLAR) in Enterococcus Species in a Tertiary Care Centre. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Revathy. S, Sridharan. K. S, Elumala. A. S, Umasekar. Phenotypic Detection of High-Level Aminoglycoside Resistance (HLAR) in Enterococcus Species in a Tertiary Care Centre. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8931. |
Introduction
The member of genus Enterococcus is gram-positive cocci that are arranged in pairs or as short chains. The enterococci are facultative anaerobes that grow at temperatures ranging from 10 to 45°C with optimum growth at 35°C. They grow in media containing 6.5 percent NaCl and hydrolyze esculin in presence of 40 percent bile salts (bile esculine (BE) medium). Some species are motile.1 Enterococci is predominantly inhabit gastro-intestinal tract and are less commonly found in other sites such as the genitourinary tract and the oral cavity. Enterococci have increasingly emerged as a cause of serious nosocomial and community-acquired infections. As mentioned in the 1984 National Nosocomial Infection surveillance summary listed the Enterococcus as the third most common cause of nosocomial infections, it caused approximately 10% of all such infections including 14.7% UTIs and 7% of bacteraemias.2 The increased Enterococcal diseases may be due to increase in the use of anti microbial agents to which enterococci are resistant. In our study we focus on aminoglycoside as antimicrobial agents. Antimicrobial resistance can be classified as intrinsic or acquired. Intrinsic resistance is related to inherent or natural chromosomally encoded characteristics present in all or most of the Enterococci. In contrast, occurrence of acquired resistance is more variable resulting from either mutation in existing DNA or acquisition of new genetic determinants found in plasmids or transposons.3 Because of the poor activity of several antimicrobial agents against Enterococci due to intrinsic resistance, the recommended therapy for serious infections (i.e. endocarditis, meningitis, and other systemic infections, especially in immunocompromised patients) includes a combination of a ‘cell-wall-active agent, such as a beta lactam (usually penicillin) or Vancomycin, combined with an aminoglycoside (usually gentamicin or streptomycin)3. These combinations overcome the intrinsic resistance exhibited by the Enterococci and a synergistic bactericidal effect is generally achieved since the cell- wall active agent facilitates the intracellular penetration of the aminoglycoside.2 The efficacy of the above combinations has been compromised by the emergence of enterococcal strains displaying multiple antibiotic resistances, including high-level resistance to aminoglycosides, and resistance to penicillins or glycopeptides.1 In addition to the intrinsic resistance traits, Enterococci have acquired different genetic determinants that confer resistance to several classes of antimicrobial drugs.3
Strains expressing acquired HLR to aminoglycosides usually have minimal inhibitory concentration (MIC) >2000µg/ml and cannot be detected by diffusion tests with conventional disks. Special tests using high-content gentamicin and streptomycin disks 4, as well as a single dilution method, were developed to screen for this type of resistance.5 This study was done with the intention of determining High Level Aminoglycoside Resistance from the Enterococcal species isolated from various clinical sample by Kirby-Bauer disk diffusion method and Single concentration agar dilution test using the drugs gentamicin and streptomycin.
Isolates that are resistant to the cell-wall-active agents or have HLR to aminoglycosides are resistant to the synergistic effects of combination therapy and constitute an even more serious problem concerning the effective management of Enterococcal infections. These resistance traits are of particular clinical relevance as they confer resistance to agents used in the treatment of serious Enterococcal infections and can abolish the activity of the therapeutic regimes with proven bactericidal activity against Enterococci. Therefore, the detection of resistance to these groups of antimicrobial agents is important.
Materials and Methods
Chemicals used for preparing media were analytical grade and commercially available drugs were used for preparation of antibiotic impregnated disc. The quality of disc is checked before subjected to the study. Enterococcal isolates reported from various clinical samples in a tertiary care center was collected consecutively during the study period between 18.01.08 and 25.02.08. The breakup of samples was urine-42 (n=42), Exudate-26 (n=26), Blood-4 (n=4) and Others-4 (n=4), which included bile-2; tissue-1 and ascetic fluid-1.Total number of isolate collected was 76. All the isolates belong to genus Enterococci was identified by colony morphology, Gram stain, catalase production, bile esculin hydrolysis, growth in 6.5% NaCl broth and speciated by fermentation of pyruvate, mannitol. There were 55 Enterococcus faecalis, 21 Enterococcus faecium. Control strains were E.faecalis ATCC 29212.
All the isolates were subjected to screening of high-level aminoglycosides resistance by Kirby-Bauer disk diffusion using indigenously prepared high content Gentamicin disk (120µg/disk) and Streptomycin (300µg/disk) this strain also subjected to single concentration agar dilution test. Agar dilution test was done with drug concentration of 500µg/ml and 2000 µg/ml of Gentamicin and 2000µg/ml of Streptomycin in Muller Hinton Agar.
Disk diffusion test
Disk diffusion test was done in Muller Hinton Agar (Himedia, batch no.M173500G) plates as per standard recommended guidelines. For disk diffusion 4 or 5 colonies of Enterococci was inoculated in nutrient broth and incubated at 37°C till turbidity matches 0.5% McFarland standard, Lawn culture made in Muller Hinton Agar plates and high content disk were placed. The plates were incubated at 37°C for 24 hours.
Reading was taken after 24 hours and 48 hours. A zone of inhibition of sizes less than 9mm is considered as high-level aminoglycoside resistant, 9-11 mm as intermediately susceptible and more than 11 mm as susceptible for both Gentamicin and Streptomycin.
Agar dilution test
Muller Hinton Agar was used as base medium for preparation of single high concentration drug agar dilution test. Plates were prepared according to recommended standard CLSI guidelines. For Agar dilution test plates were prepared with Gentamicin concentration of 500µg/ml, 2000 µg/ml and Streptomycin 2000µg/ml 1ml of diluted drug with appropriate concentration is added to 19 ml of molten medium. The plates were poured under sterile conditions and allowed it to dry. The dried plates were incubated for quality checking overnight. With the help of marking pen 1x1cm square boxes made over the base of the plate and numbered. Strains were tested in a plate after drying in their respective boxes. For inoculation 4 or 5 colonies were inoculated in nutrient broth and incubated till turbidity matches 0.5 Mc Farland standard and appropriate dilutions were made for final concentration of 106 CFU/ml. Spot inoculations was done in the corresponding boxes and incubated overnight. Reading was taken after 24 hours. The number of boxes showing visible growth were noted down and considered as High-Level Gentamicin Resistant (HLGR) and High Level Streptomycin Resistant (HLSR) strain.
Result and Discussion
A total of 76 strains were isolated and identified as Enterococcus spp, from several clinical samples. Two species were identified; strains were E.faecalis and were E.faecium. We had 55 Enterococcus faecalis (72.36%), and 21 Enterococcus faecium (27.63%). Most of them, E.faecalis 25/42(%) and 17/42(%) E.faecium was isolated from urine samples. E.faecalis accounted for a greater percentage of isolates from urine and wound cultures than from fluid and blood cultures.
Antimicrobial resistance in Enterococcus has been increasing prevalence mainly in hospitalized patients. In the present study, there was a preponderance of samples received from females 40 (52.6%) than males 36 (47.3%), the probable reason for this could be urine specimens were largest in number 42 (55.26%) compared to others and also the fact that UTI is more common in females. 10% of all UTIs and 16% of nosocomial UTIs are caused by Enterococcal species and among the Enterococcal isolates 80-90% of the isolates were E.faecalis and 5-10% E.faecium.6 Total number of I strain enrolled in the study was 76 obtained from various specimens. Enterococcus faecalis was the predominant species (n=55) followed by Enterococcus faecium (n=21). Predominant specimen was urine (n=42) followed by exudates (n=26). Gentamicin and streptomycin were the drugs used for detection of high-level aminoglycosides resistance as these drugs are used frequently in the clinical settings. High content gentamicin and streptomycin disk and single concentration agar dilution methods were used for detection. In case of high content disk diffusion method, the number of strains that were resistant to high content Gentamicin (120µg) disk was 32 (42.10%) and Streptomycin (300µg) was 27 (35.52%).
In case of single concentration agar dilution method Among the 76 isolates 9 (11.84%) were resistant to both gentamicin 2000 µg/ml and streptomycin 2000µg/ml by agar dilution. A total of 18 (23.68%) strains showed high-level gentamicin resistance out of which 5 (6.57%) were according to CLSI definitions showing resistance to 120µg gentamicin disk in the disk diffusion method and also agar dilution method with upto 500µg/ml of gentamicin. The rest of the 13(17.10%) strains had a MIC > 2000µg/ml for gentamicin. High-level resistance to streptomycin alone was recorded in 9 (11.84%) strains, which were not HLGR. That is 11.84% of Enterococcal isolates were both High Level Gentamicin Resistant and High Level Streptomycin Resistant and 17.10% were only High Level Gentamicin Resistant. E.faecalis is the species, which predominantly exhibited resistant pattern. The increased Enterococcal diseases may be due to increase in the use of anti microbial agents to which enterococci are resistant, this may cause UTIs, bacteraemia, endocarditis, meningitis, and other systemic infections, especially in immuno-compromised patients, The treatment of choice for such infections is usually the synergic combination of penicillin or a glycopeptide with an aminoglycoside, most commonly gentamicin. The efficacy of the above combinations has been compromised by the emergence of enterococcal strains displaying multiple antibiotic resistances, including high-level resistance to aminoglycosides, and resistance to penicillins or glycopeptides. Enterococci have acquired different genetic determinants that confer resistance to several classes of antimicrobial drugs and even overcomes synergistic effect also.
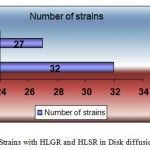 |
Figure 1a: Strains with HLGR and HLSR in Disk diffusion method. Click here to View figure |
 |
Figure 1: Agar dilution reconfirmation HLSR. Click here to View figure |
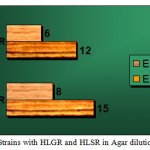 |
Figure 2: Strains with HLGR and HLSR in Agar dilution. Click here to View figure |
Table 1: Disk diffusion method:
| Resistance by disk diffusion | Number of strains | Percentage |
| HLGR | 32 | 42.10 |
| HLSR | 27 | 35.52 |
Table 2: Strains that are HLAR:
| Strains | Agar dilution | |
| 500 µg | 2000 µg | |
| HLGR | 28 (36.8%) | 23 (30.26%) |
| HLSR | *** | 18 (23.68%) |
Table 3: Strains with HLGR and HLSR in Agar dilution.
| SPECIES | HLGR | HLSR | TOTAL HLAR STRAINS |
| E.faecalis | 15 | 12 | 27 |
| E.faecium | 8 | 6 | 15 |
Conclusion
Therefore screening for high-level aminoglycoside resistance must be included in routine antibiotic susceptibility reporting for Enterococcal isolates, especially from blood as high-level resistant strains does not show synergism with cell wall acting antibiotics like penicillin group and vancomycin.
Acknowledgement
We are thankful to Department of Microbiology of Ramachandra University for providing the necessary lab facilities to carryout my work.
Reference
- Topley and Wilson, Microbiology and Microbial infections, Bacteriology volume 2, 10th edition.
- M.Louie, A.E. Simor, S. Szeto, M.Patel, B.kreiswirth, and D.E. Low Susceptibility testing of clinical isolates of Enterococcus faecium and Enterococcus faecalis. Journal of Clinical Microbiology, Jan, 1992. p. 41-45.
- Sahm. D.F and Torres, C. 1988. High-content aminoglycoside disks for determining aminoglycoside-penicillin synergy against Enterococcus faecalis. J Clin Microbiol, 26, 257-60.
- Susan Adams et al. Resistance to six aminoglycosidic aminocyclitol antibiotics among Enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrobial agents and chemotherapy, sept, 1977, p. 401-405.
- Murray, B.E., Singh, K.V., et al. 1990. comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol, 28, 2059-63.
- Facklam, R.R. and Carvalho, M.G.S. and Teixeira, L.M. 2002. History, taxonomy, biochemical characteristics, and antibiotic susceptibility testing of enterococci. In: Gilmore, M.S., Clewell, D.B., et al. (eds), the enterococci: pathogenesis, molecular biology and antibiotic resistance, Washington, DC: ASM press, 1-54.
- B E Murray, the life and times of the Enterococcus, Clini Microbiol Rev, 1990 January; 3(1): 46-65.
- Daniel F. Sahm and Carmen Torres, Effect of medium and inoculum variation on screening for high-level aminoglycoside resistance in Enterococcus faecalis. Journal of Clinical Microbiology, Feb.1988, p.250-256.
- Sifuentes-Osornio J, Ponce de Leon A, Munoz-Trejo T, et al. Antimicrobial susceptibility patterns and high-level gentamicin resistance, among enterococci isolated in Mexican tertiary care center. Rev Invest Clin 1996; 48(2): 91-96.
- Agarwal, Jain Y, pathak A, Concomitant high level resistance to penicillin and aminoglycosides in enterococci at Nagpur, Central India, Indian J Med Microbiol 1999; 17:85-87.
- Facklam, R.R. and Collins, M. D. 1989. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol, 27, 731-4.
- Irving Nachamkin et al. Multiply high-level-aminoglycoside-resistant Enterococci isolated from patients in a university hospital. Journal of clinical microbiology, July 1988, p. 1287-1291.
- B J Buschelman, M J Bale, R N Jones Department of Pathology, University of Iowa College of Medicine, Iowa City. Species identification and determination of high-level aminoglycoside resistance among enterococci. Comparison study of sterile body fluid isolates, 1985-1991. Diagn Microbiol Infect Dis. 1993 Feb ;16 (2):119-22 8467622 (P,S,E,B) Cited:2
- Swenson, J.M., Ferraro, M.J., National Committee for Clinical Laboratory Standard Working Group on Enterococci. et al. 1995. Multilaboratory evaluation of screening methods for detection of high-level aminoglycoside resistance in enterococci. J Clin Microbiol, 33, 3008-18. Facklam
- David Paterson , Janet botman and Mee Len Thong, Royal Brisbane Hospital, Herston Road, Hersten, Queensland 4006. High level aminoglycoside resistance in Enterococcal blood culture isolates Comm Dis Intell 1996; 20: 532-535.
- Mondino, S.S.B., Castro, A.C.D., et al. 2003. Phenotypic and genotypic characterization of clinical and intestinal enterococci isolated from inpatients and outpatients in two Brazilian hospitals. Microb Drug Res, 9, 2, 167-74.
- Jose Arellano Galindo M.Sc Yamin GArzon Tejada, Silvia Giono Cerezo ph.D, olga Mateos Salazar, Efren Alberto Pichardo Reyes M.D. High level aminoglycoside resistance enterococcus spp in a tertiary care hospital in Mexico. Rev Electron Biomed / Electron J BIomed 2005; 1: 40-45
- Raffaele Zarrilli et al. Molecular epidemiology of high-level aminoglycoside-resistant enterococci isolated from patients in a university hospital in southern Italy. Journal of Antimicrobial Chemotherapy 2005 56 (5): 827-835
- M.G. Karmarkar ,Edwin S.Gershom, Santosh Kaul, Aparna A. Mankeshwar and P.R. Mehta. Study of drug resistance in clinical isolates of Enterococci with special reference high level aminoglycoside resistance, β lactamase production and lateral transfer of drug resistance. Volume 1289, April 2006, pages 111-114.
- M.J.Weinbren, A.P. Johnson and N. Woodford. Defining high-level gentamicin resistance in enterococci. Journal of antimicrobial chemotherapy (2000) 45, 401-412.
- Blaimont, B., Charlier, J. and Wauters, G. 1995. Comparative distribution of Enterococcus species in faeces and clinical samples. Microb Ecol Health Dis, 8, 87-92.

This work is licensed under a Creative Commons Attribution 4.0 International License.





