How to Cite | Publication History | PlumX Article Matrix
Process Optimization of Pantoprazole Sodium Enteric Coated Tablets
V. Ravichandhran
Department of Pharmaceutics,Vels College of Pharmacy, Pallavaram, Chennai India.
Corresponding Author E-mail: sen03mpharm@gmail.com
ABSTRACT: Pantoprazole is proton pump inhibitor, which prevent the production of acid in the stomach. Pantoprazole sodium enteric coated tablets were prepared by direct compression method. During this study the process parameters like granulation process, Compression process and Coating process are optimized by conducting the study with various blending time, blending speed, compression machine speed, pan speed, spray rate, spray gun distance to tablet bed, atomizing air pressure. Based on the evaluation results of various trials the optimum process parameters are selected (bending time-23min,blending speed- 6rpm, compression speed-30rpm, pan speed-9rpm, spray rate- 70ml/gun/min, spray gun distance to tablet bed- 24cm and atomizing air pressure-6kg/cm2). By using this optimized parameters the final batch was prepared it was subjected to evaluation. The results are correlated with the standard specified limits.
KEYWORDS: pantoprazole sodium; process optimization; Granulation process; Compression process
Download this article as:| Copy the following to cite this article: Ravichandhran. V. Process Optimization of Pantoprazole Sodium Enteric Coated Tablets. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Ravichandhran. V. Process Optimization of Pantoprazole Sodium Enteric Coated Tablets. Biosci Biotechnol Res Asia 2009;6(2). Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8736. |
Introduction
Optimization is the discipline of adjusting a process so as to optimize some specified set of parameters without violating some constraint. The most common goals are minimizing cost, maximizing output, and/or efficiency. This is one of the major quantitative tools in industrial decision making. It is a useful tools to quantitative a formulation that has been qualitatively determined. The development of formulation or process a series of logical steps are performed changening one variable at a time until a satisfactory and best formulation or process is produced
Objective
The major objective of the present investigation is to optimize the process parameters during preparation of Pantoprozole sodium enteric coated tablets. This work involves two important steps. First step is to study the Granulation, Compression & Coating Processes and parameters. Second step is going to optimize those parameters to be effected by taking different trial batches.
Materials and Methods
Pantoprazole sodium was prepared by direct compression method by using Hypromellose, Mannitol Crospovidone, Methacrylic acid co polymer type ‘c’ USP, Polyethylene Glycol, Calcium stearate and Titanium dioxide. Optimization of granulation parameters at blending stage (Mixing speed and Mixing time), tablet compression parameters (Compression machine speed), tablet coating parameters (Spray rate, Pan speed, Spray gun distance to tablet bed, Atomizing air pressure )were optimized by conducting various trials(BI,BII,BIII)
Characterization of tablets
The properties of enteric coated tablet, such as thickness, hardness, friability, weight variation and content uniformity were determined using reported procedure.
In vitro release studies
The in vitro dissolution studies were performed using USP dissolution apparatus (paddle) type at 100 rpm. The dissolution medium consisted of 0.1 N hydrochloric acid for first 2h and subsequent 1h in phosphate buffer pH 6.8
Results and Discussion
The process optimization was determined by different parameters.
Granulation parameters
The optimum blending time was selected by carrying out blending at different duration of time i.e. 18min, 20min, 23min, and 25min, out of that optimum blending time was found to be 23min. During this study, the % drug content in 18min, 20min showed very lesser when compared to 23min and 25 minuts.There is no significant difference in % drug content in 23 and 25 minuts. So optimum blending is time 23min. The optimum blending speed was selected by carrying out blending at different rotation per minute (RPM) namely 4rpm, 6rpm, and 8rpm; out of these the optimum blending Speed was found to be 6rpm. The % drug content was very less with blending speed 4 rpm when compared to 6rpm and 8rpm.In the blending speed of 6rpm and 8rpm there was no significant changes in the % drug content. So optimum blending speed is 6rpm.
Compression parameters
The optimum compression machine speed was selected by running the machine at varying speed from 20 to 35 rpm. It was found that 30 rpm was the optimum speed because above that quality of product is not consistent.
Coating parameters.
The optimum coating pan speed was selected by running the coating pan at varying rpm from 8 to 10 rpm. When it was subjected to 8 rpm, it showed the differences in average weight in all the three batches. Where as in 9 rpm and 10 rpm, the results were correlated with the specification. So optimum coating pan speed is 9 rpm. The optimum atomizing air pressure was selected by continuously changing the air pressure from 5 to 7 kg/cm2, out of these optimum atomization air pressures was found to be 6 kg/cm2. In 5kg/cm2 and 7 kg/cm2 atomizing air pressure, the average weight of tablet showed significant differences. When it was subjected at 6kg/cm2 atomizing air pressure, the results are correlated with the specifications. The optimum spray rate was selected by changing the spray rate from 50 to70 (ml/gun/min), out of these optimum spray rate was found to be 70 ml/gun/min. Since the results (physical parameter evaluation) obtained with 70 ml/gun/min correlated with the specification when compared to the results obtained with 50 (ml/gun/min) and 60 ml/gun/min. By using the above optimized parameters, the Pantoprazole sodium enteric coated tablets were prepared. Then it was subjected to dissolution study. The dissolution data obtained with all the three batches correlated with the standard specified limits.
Table 1: Optimization of blending time.
| Sample | % Drug content | |||||||||||
| BI | BII | BIII | ||||||||||
| Blending time | Blending time | Blending time | ||||||||||
| 18 min | 20 min | 23 min | 25 min | 18 min | 20 min | 23 min | 25 min | 18 min | 20 min | 23 min | 25 min | |
| 1 | 92.8 | 95.9 | 98.3 | 98.2 | 92.6 | 95.4 | 98.4 | 98.2 | 92.7 | 95.7 | 98.4 | 98.6 |
| 2 | 92.9 | 94.8 | 98.6 | 98.8 | 92.9 | 95.5 | 98.4 | 98.4 | 92.8 | 95.3 | 98.6 | 98.7 |
| 3 | 92.7 | 95.2 | 98.8 | 98.7 | 92.6 | 95.6 | 98.5 | 98.6 | 92.7 | 95.5 | 98.5 | 98.3 |
| Avg. | 92.8 | 95.3 | 98.6 | 98.6 | 92.7 | 95.5 | 98.4 | 98.4 | 92.7 | 95.5 | 98.5 | 98.5 |
Table 2: Optimization of blending speed.
| Sample | % Drug content | ||||||||
| BI | BII | BIII | |||||||
| Blending speed | Blending speed | Blending speed | |||||||
| 4rpm | 6 rpm | 8 rpm | 4 rpm | 6 rpm | 8 rpm | 4 rpm | 6 rpm | 8 rpm | |
| 1 | 92.3 | 98.4 | 98.9 | 92.5 | 98.3 | 98.4 | 92.1 | 98.5 | 98.6 |
| 2 | 92.4 | 98.4 | 98.2 | 92.5 | 98.8 | 98.5 | 92.1 | 98.4 | 98.2 |
| 3 | 92.2 | 98.9 | 98.6 | 92.4 | 98.4 | 98.6 | 92.2 | 98.9 | 98.9 |
| Avg. | 92.3 | 98.6 | 98.6 | 92.5 | 98.5 | 98.5 | 92.1 | 98.6 | 98.6 |
Table no.3 Optimization of compression speed in batch I
| Parameter | Limit | Machine speed (RPM) | |||||||
| 20 | 25 | 30 | 35 | ||||||
| LHS | RHS | LHS | RHS | LHS | RHS | LHS | RHS | ||
| Appearance | White to off white coloured circular tablets with plain surfaces on both sides | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Average Weight 20 tablets(mg) | 350mg ± 4%
(336 mg -364 mg) |
347 | 348 | 349 | 350 | 350 | 351 | 344 | 343 |
| Thickness(mm) | 3mm±0.2mm
(2.80mm-3.20mm) |
2.96 | 2.95 | 2.97 | 2.96 | 3.12 | 3.13 | 3.22 | 3.23 |
| Hardness(kg/cm2) | NLT 2.5 Kg/cm2 | 4 | 4 | 4 | 4 | 5 | 5 | 3 | 3 |
| Disintegration time(min) | NMT 12min | 3’45” | 3’50” | 3’50” | 3’55” | 4’05” | 4’07” | 3’29” | 3’31” |
Table 4: Optimization of compression speed in batch II.
| Parameter | Limit | Machine speed (RPM) | |||||||
| 20 | 25 | 30 | 35 | ||||||
| LHS | RHS | LHS | RHS | LHS | RHS | LHS | RHS | ||
| Appearance | White to off white coloured circular tablets with plain surfaces on both sides | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Average Weight 20 tablets(mg) | 350mg ± 4%
(336 mg -364 mg) |
348 | 348 | 349 | 350 | 351 | 352 | 343 | 343 |
| Thickness(mm) | 3mm±0.2mm
(2.80mm-3.20mm) |
3.0 | 2.98 | 3.02 | 3.05 | 3.13 | 3.13 | 3.22 | 3.24 |
| Hardness(kg/cm2) | NLT 2.5 Kg/cm2 | 4 | 5 | 5 | 4 | 5 | 5 | 3 | 4 |
| Disintegration time(min) | NMT 12min | 3’43” | 3’47” | 3’51” | 3’54” | 4’04” | 4’09” | 3’30” | 3’31” |
Table 5: Optimization of compression speed in batch III
| Parameter | Limit | Machine speed (RPM) | |||||||
| 20 | 25 | 30 | 35 | ||||||
| LHS | RHS | LHS | RHS | LHS | RHS | LHS | RHS | ||
| Appearance | White to off white coloured circular tablets with plain surfaces on both sides | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Average Weight 20 tablets(mg) | 350mg ± 4%
(336 mg -364 mg) |
346 | 348 | 349 | 351 | 350 | 351 | 342 | 343 |
| Thickness(mm) | 3mm±0.2mm
(2.80mm-3.20mm) |
2.96 | 2.94 | 2.95 | 2.96 | 3.10 | 3.13 | 3.23 | 3.23 |
| Hardness(kg/cm2) | NLT 2.5 Kg/cm2 | 5 | 4 | 4 | 4 | 5 | 5 | 3 | 3 |
| Disintegration time(min) | NMT 12min | 3’46” | 3’48” | 3’50” | 3’52” | 4’03” | 4’06” | 3’27” | 3’29” |
Table 6: Optimization of pan speed.
| Parameter | Limit | Pan speed (RPM) | ||||||||
| Batch 1 | Batch 2 | Batch 3 | ||||||||
| 8 | 9 | 10 | 8 | 9 | 10 | 8 | 9 | 10 | ||
| Appearance | Off white to yellow | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Thickness
(mm) |
3.5mm±0.2mm
(3.30mm-3.70mm) |
3.31 | 3.52 | 3.50 | 3.2 | 3.54 | 3.52 | 3.39 | 3.53 | 3.50 |
| Average Weight 20 tablets(mg) | 375mg ± 4%
(360mg – 390mg) |
370 | 375 | 375 | 371 | 377 | 378 | 372 | 375 | 376 |
Table 7: Optimization of Atomization air pressure.
| Parameter | Limit | Atomization air pressure (kg/cm2) | ||||||||
| Batch 1 | Batch 2 | Batch 3 | ||||||||
| 5 | 6 | 7 | 5 | 6 | 7 | 5 | 6 | 7 | ||
| Appearance | Off white to yellow | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Thickness (mm) | 3.5mm±0.2mm (3.30mm-3.70mm) | 3.5 | 3.6 | 3.8 | 3.3 | 3.6 | 3.8 | 3.4 | 3.6 | 3.8 |
| Average Weight 20 tablets (mg) | 375mg ± 4%
(360 mg -390 mg) |
372 | 375 | 380 | 371 | 376 | 381 | 371 | 374 | 380 |
Table 8: Optimization of of spray rate.
| Parameter | Limit | Spray rate(ml/gun/min) | ||||||||
| Batch 1 | Batch 2 | Batch 3 | ||||||||
| 50 | 60 | 70 | 50 | 60 | 70 | 50 | 60 | 70 | ||
| Appearance | Off white to yellow | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Thickness (mm) | 3.5mm±0.2mm (3.30mm-3.70mm) | 3.2 | 3.3 | 3.5 | 3.3 | 3.3 | 3.6 | 3.3 | 3.4 | 3.6 |
| Average Weight 20 tablets (mg) | 375mg ± 4%
(360 mg -390 mg) |
371 | 373 | 375 | 372 | 374 | 375 | 371 | 374 | 375 |
Table 9: Optimization of spray gun distance.
| Parameter | Limit | Spray gun distance(cm) | ||||||||
| Batch 1 | Batch 2 | Batch 3 | ||||||||
| 22 | 23 | 24 | 22 | 23 | 24 | 22 | 23 | 24 | ||
| Appearance | Off white to yellow | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| Thickness (mm) | 3.5mm±0.2mm (3.30mm-3.70mm) | 3.9 | 3.9 | 3.6 | 4.0 | 3.9 | 3.6 | 3.9 | 3.8 | 3.5 |
| Average Weight 20 tablets(mg) | 375mg ± 4%
(360 mg -390 mg) |
381 | 380 | 375 | 383 | 381 | 376 | 379 | 379 | 375 |
Table 10: Dissolution profile pantoprazole sodium enteric coated tablets BI
|
S.No. |
% Cumulative Drug release | ||||
| Time interval(min) | |||||
| 15 | 20 | 30 | 45 | 60 | |
| 1 | 35.1 | 46.5 | 63.2 | 79.3 | 98.1 |
| 2 | 35.2 | 46.9 | 63.2 | 79.3 | 98.8 |
| 3 | 35.5 | 46.5 | 63.5 | 79.9 | 98.6 |
| 4 | 35.3 | 46.6 | 63.3 | 79.5 | 98.6 |
| 5 | 35.5 | 46.3 | 63.4 | 79.6 | 98.5 |
| 6 | 35.9 | 46.4 | 63.9 | 79.9 | 98.3 |
Table 11: Dissolution profile pantoprazole sodium enteric coated tablets BII
| S.No. | % Cumulative Drug release | ||||
| Time interval(min) | |||||
| 15 | 20 | 30 | 45 | 60 | |
| 1 | 37.2 | 48.1 | 64.2 | 80.1 | 98.3 |
| 2 | 37.4 | 47.9 | 64.5 | 79.8 | 98.4 |
| 3 | 37.7 | 47.8 | 64.4 | 80.2 | 98.6 |
| 4 | 37.2 | 48.2 | 64.7 | 80.5 | 98.2 |
| 5 | 37.6 | 47.8 | 64.3 | 79.9 | 98.5 |
| 6 | 37.5 | 48.1 | 64.4 | 80.1 | 98.6 |
Table 12: Dissolution profile pantoprazole sodium enteric coated tablets BIII.
| S. No. | % Cumulative Drug release | ||||
| Time interval(min) | |||||
| 15 | 20 | 30 | 45 | 60 | |
| 1 | 36.5 | 49.1 | 66.2 | 78.1 | 98.2 |
| 2 | 36.7 | 49.9 | 66.4 | 79.2 | 98.6 |
| 3 | 36.4 | 49.3 | 66.7 | 79.2 | 98.4 |
| 4 | 36.6 | 49.4 | 66.7 | 79.1 | 98.6 |
| 5 | 36.2 | 49.8 | 66.3 | 79.9 | 98.7 |
| 6 | 36.5 | 49.4 | 66.4 | 79.1 | 98.9 |
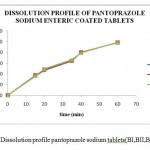 |
Figure 1: Dissolution profile pantoprazole sodium tablets(BI,BII,BIII). |
Conclusion
The process optimization for the preparation of pantoprazole sodium enteric coated tablets was done and the Optimum blending time 23min, Optimum blending speed 6 rpm, Optimum compression speed 30 rpm, Optimum coating pan speed 9 rpm, Optimum coating spray rate 70 ml/gun/min, Optimum coating spray gun distance to tablet bed 24 cm, Optimum coating atomizing air pressure 6 kg/cm2 were selected as optimized parameters for the production of Pantoprazole sodium enteric coated tablets . The dissolution data obtained with all the three batches correlated with the standard specified limits which were prepared by using the optimized parameters.
References
- Leon Lachman, Herbert A.Liberman Lachman, Joseph L, Kaning “theory and practice of industrial pharmacy”, page no. 171-198, 293-373.l.
- Liberman Lachman “Tablets Dosage forms” volumeII&III page no. 274-348.
- Liberman Lachman “pharmaceutical dosage forms” volume II, page no 366-368.
- JVV MCGINITY Aqeous polymeric coating for pharmaceutical dosage forms – 1997, page no 81-95,227-267
- Www. Wikepedia.com
- Devault G., Chassary O., Schmitt H., “international validation of health- related quality of life questionnaire in patients with erosive gastro-oesophageal reflux disease” Aliment pharmacoltger.2009 mar 15.29 (6).
- KD.Bardhan., Stanghellini.V., Gatz G., “international validation of request in patients with endoscopy negative gastro-oesophageal reflux disease”2007 may 4.
- Unsalan.S., Rollas.S., “Determination and validation of ketoprofen, pantoprazole and valsartan together in human plasma by high perforanceliquid chromatography”.
- Raffin Rp., Guterres SS., Jornada DS., “pantoprazole sodium effect of scale of production and validation”.
- Filipe A., Almedia S., Franco., Spinola AC., “Bioequilence study of two enteric coated formulation of pantoprazole sodium” Arzneeimittel forschung 2008.
- Howden CW., Ballard ED., Koch FK., “Release rate of pump inhibitors in patients with GERD” J Clin Gasstroenterol, 2009 April, 43(4).
- Hoggan D., Pratha V., Riff D., “Oral pantoprazole in the form of granules or tablets are pharmacodynimically eqivalent in supressing acid output in patients with gasstro-oesophageal reflux disease”.
- Pohlmann AR., Guterres SS., “Prepartion, characterisation and in vivo anti-ulcer evaluation of pantoprazole –loaded micropaticles”.
- Santos SR., Mrrtins VL., Araujo MC., “A flow- injection biamperometric method for determination of pantoprazole in pharmaceutical tablets”

This work is licensed under a Creative Commons Attribution 4.0 International License.





