How to Cite | Publication History | PlumX Article Matrix
Samira Omar Abu Bakr Balubaid
Department of Biology, Faculty of Science, King Abdul Aziz University, Jeddah Saudi Arabia.
ABSTRACT: The aim of this study was to identify the impact of the new antibiotic “Maxipime” (Cefepime hydrochlorid) “, which belongs to cephalosporin group on the development of composition of the liver in fetuses, infants’ white rats during pregnancy and lactation. Using Maxipime drug during the different phases of pregnancy, invagination (0-7 day) ,organs formation (7-14 day) and growth period (14-21 day) led to a lot of pathological changes in the fetal liver and these changes were obviously appeared in the first and second trimester. The histological examination of rat liver during lactation showed a decrease in the impact of the drug at the age of one week of lactation, and this effect restricted on limited areas at 14 days age and the liver with normal hepatic structure at the end of weaning age .It was evident in the low intensity of these effects during the lactation period in the histological sections of the maternal liver as restricted to limited areas, while included most of the liver tissue during pregnancy. Therefore, it is recommended to: never provide Maxipime during gestation especially during the first and second period and treatment with Maxipime should not be used during lactation before the 14th day of delivery.
KEYWORDS: pregnancy and lactation liver; albino rats; Maxipime;
Download this article as:| Copy the following to cite this article: Balubaid. S. O. A. B. Study the Adverse Effects of Antibiotic Maxipime (Cefepime Hydrochloride) on the Evolution of Composition of the Liver in Albino Rats During Pregnancy and Lactation. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Balubaid. S. O. A. B. Study the Adverse Effects of Antibiotic Maxipime (Cefepime Hydrochloride) on the Evolution of Composition of the Liver in Albino Rats During Pregnancy and Lactation. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8625 |
Introduction
The pregnant women took 900 different types of drugs and stated that these drugs caused embryonic deformations as they pass through placenta and breast milk.1 This wide using led to resistance development to these drugs enhancing scientists to discover new and selected antibiotics. Maxipime (Cefepime hydrochlorid) is considered one of the recent fourth generations of Cefepime group that was allowed to use it in Saudi Arabia for the treatment of acute and chronic inflammations. Maxipime stop the formation of the bacterial cell surface in the inflammations that other antibiotics could not treat those2 and is the most active drug against positive and negative/gram/ bacteria, thus it used in the treatment of inflammations of respiratory system, abdomen and pelvic region.3 This drug is stable against hydro decaying and has a wide activity against intestinal bacteria which resist the first group of cephalosporins and niceria and cause the inflammations of reproductive system, cerebral membranes. Maxipime is concentrated in the plasma after half hour of injection and appeared in the body fluids as urine, blood and milk as well as the body tissues.4 The effect of Cefotaxime on the functions of liver, kidney, blood and respiratory system as well as on the central nervous system was studied by,5 they provided young rats with chronic dose of the drug and did not find any deviations in the functions of liver or kidney and no changes in the blood counting or respiratory nor nervous systems. Moreover, no embryonic toxicity or mutations or deformations were observed but only some allergic symptoms were recorded.
Some experiments were carried out by6 on the mice to investigate the transferring of the antibiotic Ceftazidime through placenta to the embryo. They found that the transfer rate of the drug increased with pregnancy progressing. Another study was performed by7 to evaluate the cyclic usage of antibiotics by women before, during and after pregnancy to resist cultures of microbes in the vagina and rectum, he provided women with some antibiotics during and after delivery where Ampicllin and Cefazolin was the most used drug about 49 and 28%, respectively. Thus, scientists confirmed to provide penicillin before and after delivery as a protective drug against microscopic organisms.8 Mentioned that peritoneal injection of male and female mice with Cefpirome-sulfate by different doses (200, 400 and 800 mg/kg/day), 60 days before and during mating for males, while females were injected from the 14th day before mating to the 7th day of pregnancy. Females were dissected at the 21st day of pregnancy where no died or deformed embryos were recorded, also there were no difference in the fertility or sexual ability for male or female. However, a decrease in the body weight and enlarged caecum were noticed.9 Determined the toxicity of Cefepime dihydrochlorid on the female reproductive system after providing mice with subcutaneously daily doses (150, 500 and 1000 mg/kg for 63 days) before mating, during mating, during pregnancy and lactation for 14 days. Softening stool, increasing caecum size, decreasing in the food consumption rate, hair falling and inflammations in the injected site were recorded. In the other hand, this drug did not affect the organization, growth, behavior and activity of the treated mice.In an experiment carried by10 to compare between the curial effect of Ceftazidime, Cefepine and Cefepime on the treatment of the tuberculosis. They found that the curial effect of Ceftazidime, Cefepine and Cefepime was 60, 76 and 95%, respectively. The curial dose for tuberculosis patient was about 1-2 g/ 12h for 10-14 days and not less than 5 days. They advised to notice the patient kidney functions after the treatment period.11 Provided Cefepime dose to children less than 4 months to be 30 mg/kg every 8 hours, while children more than 4 months the dose was about 50 mg/kg every 12 hours infected with inflammation, as it is the best drug with a wide range of activity against a negative gram bacteria.12 Examined the effect of hyperbaric oxygen (HBO) alone and together with Cefepime on the rat liver, they found significant decrease in the rates of hepatic enzyme activity, hepatocytes decaying, intracellular and extracellular fat filtration in the treated group with HBO. However, most liver sections appeared structurally normal in the group treated with HBO+Cef. Also13 tested these results before when they provided mice with oral dose of 40 mg/kg of Cefepime (cephalosporin) for 14 days and they found that this dose did not affect the rate, activity and efficiency of hepatic enzymes indicating the liver resistance to the drug toxicity. The findings of14 suggested that the combined therapy of cefepime + amikacin (Potentox) showed significant free radical with scavenging property which may contribute to decrease the aminoglycoside induced liver injury.
Due to the abundance of antibiotics and increasing in its use, as in sometimes pregnant woman and infant may have to use the drug “Maxipime” for the treatment of diseases that the rest of the antibiotics cannot treated and due to lack of scientific research about it. So the aim of this study was to identify the impact of the new antibiotic Maxipime (Cefepime hydro chloride) on the evolution of the composition of the liver embryos and baby albino rats during pregnancy and lactation.
Materials and Methods
The maintenance of the animals was in full compliance with the standard laboratory animals care protocols approved by Institutional Animal Care and Use Committee (IACUC).Pregnant female albino mice were used in this study after be sure of fertilization by examining the mucosal vaginas, then they were divided into two divisions:
1-The first division where females treated during gestation and then divided into two groups:
Group A: Control group injected with distilled water equivalent to the drug dose and dissected at the 20th day to obtain embryos and liver specimens.
Group B: Treated group injected with a curial dose 0.07 of “Maxipime (Goodman and Gilman, 2001) and divided into: Sub-group (1): treated during the first week (0-7 days) of the gestation and left to the 21st then mothers were dissected to obtain embryos.Sub-group (2): treated during the second week (8-14 days) of the gestation and left to the 21st then mothers were dissected to obtain embryos. Sub- group (3): treated during the third week (14-21 days) of the gestation and were dissected to obtain embryos.
2-The second division was treated during lactation and divided into two groups:
Group C: Control mothers group injected with a dose of distilled water equivalent to that of the drug dose .Then embryos were taken at the ages of 7, 14 and 21 days where liver extracted for histological examination.
Group D: Treated mothers group treated with curial dose from the first day of delivery to the 6th day, then embryos were taken at ages of 7, 14 and 21 days where liver extracted for histological examination.
Methods
1-Pregnant and lactating mothers as well as embryos were weighed at all groups and the number of the embryos was recorded.
2-Mothers were dissected to investigate embryo morphologically and liver specimens were taken from pregnant and lactating mothers as well as embryos and infants according to the chosen ages: For light microscopic studies, specimens were fixed in 10% neutral formalin and the standard procedures of dehydration, clearing and embedding in wax were followed. They were sectioned at 3-5 µm and stained with hematoxylin and eosin (Drury and Wallington1980). For electron microscopic study, parts with 1 mm² thickness fixed in glutaraldehyde 4 ºC for 24 h followed that washing in the phosphate buffer (0.1 n) ,post fixation in osmium tetra oxide 1% also dehydration and then embedded in mixture of Epon and epon oralolite, getting ultra thin 70-50 nm sections picked up on 200 mesh naked copper grid. After being double stained with uranayl acetate and lead citrate.15 The sections were examined by Philips 400 transmission electron microscope of king Fahd Center for research. For statistical analysis: Anova-test was used to assess the significance of body weight changes between control and treated mice.16
Results
Effect on weight
Table (1) showed that, there is a significant increase in embryos weight in the group treatment during pregnancy compared with the control group, while there was a notable decrease in body weight in the group treatment during breast feeding which was significantly decrease at the age of (7 – days) while there was a significant increase in weight at ages (14,21days).
Histological investigation of mother liver during different gestation periods
The usage of Maxipime during the different gestation periods caused pathological changes in the liver tissue represented by enlarged and expansion of blood vessels with stasis of red blood corpuscles. There was also disorder in the liver architecture represented by irregular arrangement of hepatic strands, dilated hepatic sinusoids, congestion and odeama inside liver tissue which stained with pale eosin. Inflammatory cells , fat vacuoles and extracellular fat filtrations were observed in the hepatocytes (Fig.1-A), decayed and necrotic hepatocytes appeared as red spots and deformed portal spaces with congested blood vessels surrounded by lytic connective tissue were recorded (Fig.1-B).
The electron microscopy study showed change in the cell liver to oval shape with irregular cell membrane, many fatty drops and swollen, plofilated mitochondria (Fig.1-C).In addition to the increased content of the rough endoplasmic reticulum (RER), Golgi degenerated and fragmented. Also, more ribosomal content, irregular nuclear envelope, margination of chromatin around nuclear pores was recorded (Fig.1-D). Disc space was dilated and filled with proliferated hepatocytes microvilli and large Kuppfer cells (Fig.1-E). Also, dilated blood sinusoids contained endothelial cells increase in size (Fig. 1-F)
Histological investigation of mother liver during lactation period
It was observed that liver sections were darkly stained with eosin and showed less acute congestion and odeama inside the tissue than that appeared during gestation. However some sections contained congested blood vessels with ruptured walls and proliferated bile ductules in the portal spaces with abnormal structure. Kuppfer cells numbers were increased (Fig.1-G). The fat vacuoles inside the hepatocytes led to change their shape and drifting nuclei to one side of the cell. Also, congested and enlarged hepatic sinusoids filled with red blood corpuscles were observed (Fig.1-H).
Histological investigation of embryo liver during gestation period
Histological investigation of liver of embryos at (21-days) age treated in the first week of gestation showed disorder in the liver architecture, where the blood vessels expanded and some of them filled with blood. Also, there was changing in the shape of some hepatocytes as they appeared with ruptured cellular membranes and scattered nuclei. Other cells contained fat granules with extracellular fat infiltration (Fig.1-I).
Liver of embryos at (21- days) age treated in the second week of gestation showed a decrease in congestion and odeama comparing with the previous group (first group).Also, many blood sinusoids appeared empty from blood. At this age, the hepatocytes still smaller in size and number with limited fibrous regions (Fig.1-J).
Pathological changes continued in the embryo liver sections treated through last week of gestation with decreased effects restricted in limited regions.
5-Histological investigation of embryo liver during lactation period
It was observed that infant liver tissue of one week age was darkly stained with eosin due to expansion and enlargement of blood vessels especially in the portal spaces. Most hepatocytes contained fat vacuoles, others appeared as red spots with enlarged hepatic sinusoids filled with blood and lymphatic infiltration occurred.
In infant liver tissue from (14 -days) proliferation, obvious growth in the number and size of hepatocytes were recorded .Also, the bile ductules increased than previous age and the portal area with large congested blood vessel with disappearance of hepatic architecture (Fig.1-K).
At (21- days) age (weaning age), the adverse effects has decreased where the histological structure of liver was nearly normal and most hepatocytes appeared normal and arranged in strands (Fig.1-L).
Discussion
The present results showed that, there was a significantly decrease in body weight in the group treatment during breast feeding (7 – days). This is supported by17 which stated that the Sifotaxim drug led to decrease in embryo weight and this decrease rises proportionately with increasing dose and age of the fetus and he interpreted this decrease due to the lack of fetal growth as a result of the drug inhibiting effect on cell division.18 Pointed that there were some kinds of antibiotics stop the cells growth in the bone marrow .Also,19 found that the antibiotics led to disorder in the cellular functions as they play an important role in inhibition of DNA and RNA synthesis and delayed or inhibited the cell division. On the other hand, the results of this study do not agree with20 as they said that the use of a drug Cefmiteleyn hydrochloride did not lead to changes in weight of embryos.
This work showed that using Maxipime during the different gestation periods; invegination (0-7day), organs formation (7-14day), growth (14-21days) periods led to many pathological changes in the liver tissue of either mothers or embryos. The most acute changes were observed during the first and second gestation periods, this may be due to the direct effect of this drug when it was provided in the first gestation period.21 These results were in accordance with those obtained with22 who confirmed the incidence of fetal toxicity of some antibiotics when give it to mice embryos at various times during the period of invagination (1-7 days) and organs formation (8 – 14 days) which led to the death and deforming of many embryos inside the uterus, also, he confirmed the passage of the drug through the placenta and milk to the fetus where the level of drug transmission increases with time.23 Mentioned that Cefepime group speed odeama, odeama may be very serious because of a reduction in the prothrompine as a result of a disorder in the blood platelets function and this may explain the congestion and odeama inside the liver tissue in mothers, embryos and infants especially during pregnancy.24 found that some antibiotics such as Cefepime intervene in the process of correlation of ribosome with RNA, therefore, was the lack of protein formation necessary for the formation of hepatic lipid acceptor protein and this leads to the inability of triglycerides to go out of the liver and accumulation within the cells and this explained the presence of fat infiltration in this study during pregnancy and breast feeding periods which confirmed by25 which stated that any material or toxins affect the formation of the protein responsible for carrying fatty acids to the liver( apoproteins) ,it would led to the accumulation of fat special triglyceride within the hepatocytes so, the fat accumulation may be caused by a disturbance in the transfer of fat from and to the liver. It was observed in this histological investigation of the young rats’ liver during lactation, that there was an obvious decrease in the drug effect at one week age and its restriction to limited regions at 14 days age. At the weaning age, the liver appeared nearly with normal structure indicating the progressive growth of the liver and its resistance to the drug toxicity; this is in agreement with.26
Regarding the changes that appeared in the liver tissue of pregnant mothers, these may be due to the sensitivity of this phase, the hormonal changes in female body and the reduction in the level of natural immunity. This was obvious in the reduction of the effects acuteness during lactation, while they were nearly observed all over the tissue during gestation. This observation indicates the liver ability to resist the drug toxicity during lactation. These results are in accordance with those obtained by.12
The present ultra structural results agree with27 who explained that the cytoplasmic granulation in liver cells due to its increase content from rough endoplasmic reticulum, ribosome, glycogen particles and mitochondria.28 considered this increase is compensatory activation for protein synthesis in liver cell .Also,29 ascribed the proliferation of Kuppfer cells for its defense activity to engulf the lytic red blood cells as it considered defense way against poisoning and bleeding .The swelling of endothelial cells were due to its important role in inflammatory reactions, also30 agree with the present results, where he noted an increase in microvilli in Disc-space of mice infected with hepatitis.
Thus it can be recommended to: not provide Maxipime during gestation especially during the first and second periods and not to use this drug during lactation and to start treatment after the 14th day of delivery.
Acknowledgements
I thank Dr. Awatef Ali, Collage of Science – Alexandria University, for her assistance and for valuable comments on the histology.
Legends
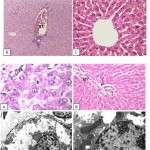 |
Figure 1: Ato l: Light micrograph of mother liver section treated during pregnant period (0-21) showing irregular hepatocytes, fatty infiltration (F) and lymphatic infiltration (arrow) (H α E x 400) .
Click here to View figure |
(B)
Light micrograph of mother liver section treated during pregnant period (0-21) showing deformed portal spaces with congested portal vein (BV) and lytic connective tissue (arrow) (H&Ex100).
(C)
Electron micrograph of mother liver section treated during the second period of pregnancy showed hepatocytes with lytic cytoplasm filled with proliferated mitochondria (M) and lipid droplets (L).
(D)
Electron micrograph of mother liver section treated during the second period of pregnancy illustrated irregular nucleus (N) with marginated heterochromatin (arrow) and dense cytoplasm filled with proliferated rough endoplasmic reticulum (RER).
(E): Electron micrograph of mother liver section treated during the second period of pregnancy illustrated wide Disc space with proliferating microvilli (MV) and large Kuppfer cell (K).
(F)
Electron micrograph of mother liver section treated during the second period of pregnancy showed hepatic sinusoid lined with large endothelial cell (E) and lytic cytoplasm contained fat globules (F).
(G)
Light micrograph of mother liver section treated during lactating period showing deformed portal spaces with congested blood vessel (BV) and proliferated bile ductules (arrows). (H α E x40).
(H)
Enlarged part from the previous figure showing dilated hepatic sinuses (H) with stasis of blood corpuscles(HαE x100) .
(I)
Light micrograph of infants liver section( 21- days) treated during pregnancy period (0-7) showing dilated blood sinusoids (H) lined with shrinked hepatocytes filled with lipid granules (arrows’) (H α E x 400 ).
(J )
Light micrograph of infants liver section( 21- days) treated during pregnancy period (7-14 ) showing thick fibrotic capsule (arrow). (H α E x100).
(K)
Light micrographs of newly born rat liver section (14-days) after delivery from treated mother during lactating period showing disappearance of hepatic architecture and portal area with large congested blood vessel (BV) (H α E x40).
(L)
Light micrographs of newly born rat liver section (21-days) after delivery from treated mother during lactating period showing nearly normal liver structure (H&E x400).
References
- Sadler T, Langnans W. Medical Embryology 8 th Edition lippincot willicinsad wilkins new york London Tokyo. 2000.
- Chambers H.F. Antimicrobical agents general consideration chapter 43 in Goodman and Gilman, tenth edition Mc Graw Hill, Newyork. 2001.
- BachmV.T,Roy I,Thadepallim H. Susceptibility of anaerobic bacteria to Cefoxitin and related compounds. Antimicrob,Agents chemother. 1977;11:912.
- Sanders C.S. Cefepime: the next generation. Clin.infect. Dis. 1993;17:369.
- Berezhinskaiam V.V, Dolgova G.V, Egorenko G.G, Svinogeeva T.P ,Zebrev A. I , Smolkina T.V, Shtegel L.A and Nikitin A.V. General toxic and organo tropic properties of cefotaxime in acute and chronic experiments .Antibiot khimioter. 1990;35:25.
- Kasabulatov K.K, Manuilov K.K and Voropaeva S.D. An animal experiment on transplacental transport of Ceftazidim at various .Antibiotic khimioter. 1993;3891:33.
- Spaetgens R.D, Ebella K,MaDRobertson S,Mucenski M and Davies H.D. perinatal antibiotic usage and changes in colonization and resiance rates of group B – stre ptococcus and other pathogens)obstet Gynecol. 2002 Sep;100:33.
- Sugiyama O, Watanabe S, Tanaka K,Toya M, Lgarashi S, Kumagai Y. perinatal and postnatal study of Cefpirome sulfate in rats .J.Toxicol. Sci. 1990;13:53.
- Kai S ,Kohmura H ,Ishikawa K , Kawano S ,Sakai A,Kuroyanagi K ,Kadota T and Takahashi N. Reproductive and developmental toxicity studies on Cefepime dihydrochloride administerd subcutanaeously to rats during the premating gestation and lactation periods. J. Antibiot. 1992;45:60.
- Jung-hung C.L, Kuo-Ming Y, Ming-Yich Pand Feng-Yee C. Efficancy of Cefepime versus Ceftazidime in the treat ment of adult pneumonia .J.Microbial Immunol Infect. 2001;34:131.
- Capparelli E, Hochwald C , Rasmussen M , Parham A and Bradl Y. Population pharmacokinetics of Cefepim in the neonate. Antimicrob Agents chemother. 2005;49:2760.
- Oter S, Edremitlioglu M,Korkmaz A,Coskun O,Kilic D,Kisa U,Yaren H and Bilgic H. Effect of hyperbaric oxygen treatment on liver function oxidative status and histology in septic rats. 2005 .
- Jordan M.S, Waller A.R,Chasseaud L.F and Barbhaiya R.H. Effect of the cephalosporin cefepime on hepatic drug metabolizing enzyme activity in rats .Toxicol let. 1994;71:63.
- Chaudhary M, Soni A, Dwivedi V.K. Fixed dose combination of cefepime plus amikacin prevent oxidative stress in liver of Mus musculus mice. Curr Clin Pharmacol. 2008;3:211.
- Drury R.A, Wallington E.A. Carleton’s Histological technigue.Oxford University Press, Oxford, New York, Toronto. 1980.
- (16)Sokal R.R and Rohlf F.J. Biometry: the principles and practice of statistics in biological research, 2nd ed. W.H. Freeman, New York. 1981.
- Al-Yousuf Z. Morphological and histological study of the impact of Sifotaxim and Tramesen on mid-brain development in the chicken embryos. Ph.D. thesis in philosophy of science. Faculty of Education, Riyadh. 2002.
- Gomes S and Fernades M. Effect of therapeutic level of doxycycline and monocline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch oral Biol. 2007;52:251.
- Olin B.R,Hebel S.K,Donbek G.E,Cremp J.L and Hulber M.K. Drug facts and compairisons 49 th Edition published by facts and comparisons. 1995;2763
- Hara K,Muraoka Y, Yoshida T , Muranaka R, Kanamo S,Hirashiba M ,Uchida H and Lkeuchi K. Reproductive and developmental toxicity studies of S-1090 ,Cefmatilen hydrochloride hydrate (1)-A study on oral administration prior And in the early stages of pregnancy in rats .J. Toxicol Sci. 2001;1:157.
- Gilbert T, Leliever –pegorier M and Merlet-benichou C. Immediat and long –term renal effect of foetal exposure to gentamicin J .pediat-nephro. 1990;l 4:445.
- Shosyreva A.M. comparative stimulation of the eubryotoxic action of some Antibiotics .union Res.Chem.Pharm .Inst Moscow . 1989;34:779.
- Sattler F.R,Weitekam M.R and Ballard J.O. potential for bleeding with the new Beta-lactam antibiotics .Ann.Intern.Med. 1986;105:924.
- Abd-Elmajid B and Jaber F. Pre and postnatal study of the developing kidney and testes of the albino rat under the effect of antibiotic “Maxipime “(Cefepime hydrochlorid), a thesis of master in science .girls college of education in Jeddah. 2009.
- Kumar V ,Cotran R and Robbins S. Basic pathology 7th edition W.B Sanders company Australia Canada. 2003 .
- Berezhinskaiam V.V, Dolgova G.V, Egorenkom G.G,Svinogeeva T.P,Zebrev A.I , Smolkina T.V,Shtegel man L.A and Nikitin A.V. General toxic and organo tropic properties of cefotaxime in acute and chronic experiments .Antibiot. 1990;35:25.
- Ghadially F .N. Ultrastrucutral pathology of the cell and matrix. A text and atlas of physiological and pathological altrations in the fine structure of cellular and extracellular components .3rd ed Butterworth. 1988;(1).
- Segner H. Response of fed and starved roach ,Rutilus rutilus ,to sublethal copper contamination .J .Fish Bio. 1987;30:423.
- Hassan F.M, El-Banhawy M .A, Mohllal M .E and Rahmy T.R. Lethality of pseudocerastes persicus fieldi venom and the effect of its sublethal dose on the liver function of envenomated rats. Egypt.J .Biochem. 1986; 4:120.
- Svoboda D, Nielson A ,Werder A and Higginson J. An electron microscopic study of viral hepatitis in mice .Am.J .Path. 1962;41:205.

This work is licensed under a Creative Commons Attribution 4.0 International License.





