How to Cite | Publication History | PlumX Article Matrix
Accumulation and translocation of trace metals in Halodule uninervis, in the Kuwait coast
A. H. Bu-Olayan and B. V. Thomas*
POB 5969, Department of Chemistry, Kuwait University, Safat-13060, Kuwait.
ABSTRACT: Trace metals run-off associated with urban and industrial development showed potential threats to seagrass in the Kuwait coastal ecosystem. Seagrass, Halodule uninervis from Doha, Duba’iyah and Nuwaseeb (Sites I-III) representing the power plant, industrial (oil fields) and recreational sites of Kuwait’s coastal waters were assessed for trace metals (Copper: Cu, Iron: Fe, Nickel: Ni and Lead: Pb) concentrations respectively. Trace metals concentrations followed an overall trend of: leaves> rhizome in H uninervis > sediments and Ni>Cu>Pb>Fe irrespective of the two seasons (summer and winter). Seasonally, trace metals in all the samples showed the sequence of July>Sept.>May> Mar.>Jan.>Nov. Among the three sites, trace metals were high in Site II followed by Sites I and III irrespective of seasons. The overall trace metals bioconcentration factor (BCF) was high in summer than in winter. BCF was higher than the translocation factor (TF) irrespective of the sampled sites and seasons. Among the sampled metals, BCF was high in Cu in both the seasons followed by other metals. However, the overall BCF in the samples was lower than the permissible limits to be defined as hyper-accumulators. Thus, the possibility of trace metals mobilization from contaminated sediment to leaves through rhizomes of H uninervis, characterized this species as a bio-indicator to trace metal pollution in the intertidal zone of Kuwaiti waters.
KEYWORDS:
Seagrass; metals accumulation; marine pollution
Download this article as:| Copy the following to cite this article: Bu-Olayan A.H, Thomas B.V.. Accumulation and translocation of trace metals in Halodule uninervis, in the Kuwait coast. Biosci Biotechnol Res Asia 2010;7(1) |
| Copy the following to cite this URL: Bu-Olayan A.H, Thomas B.V.. Accumulation and translocation of trace metals in Halodule uninervis, in the Kuwait coast. BiosciBiotechnol Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=8787 |
Introduction
Trace metals contamination pose deleterious impact on marine life when they are aggravated by their long term persistence in the marine environment. Recent industrialization, discharges from anthropogenic sources and applications involving metals have elevated their levels in the marine environment1-2. Seagrass are marine angiosperms that colonize near shore environments3. Seagrasses were found to sequester trace metals from the marine environment through both the leaves and root and these concentrations were correlated with the water column and sediments, respectively 4-6. Earlier investigators7-8 showed metals sequestered by seagrasses passed from detritus communities to secondary and tertiary consumers and thus lead to the total contamination of the marine ecosystem. Trace metals toxicity in seagrasses were found to be high in low metal tolerant species, sites subjected to domestic or sewage outfalls sites, chemical interaction and ionic speciation from sediment9-11. Many plants that accumulated >1000 mg Kg-1 of Cu, Ni, Pb or >10000 mg Kg-1 of Zn, V, Mn were categorized as metals hyper accumulators12. Researchers2, 13 observed significant trace metals mobilization between rhizome and leaves in seagrasses with translocation factor (TF) >1. Most plants translocated trace metals and nutrients from roots to leaves. Such mobility of metals was found low especially when the pH was high with clay and organic matter14. Based on the above findings, we investigated the (a) trace metals concentrations in the leaves, rhizome of H. uninervis in relation to its surrounding surface sediment (5cm depth) (b) bioconcentration (BCF) and translocation (TF) factors in H. uninervis and (c) interrelationship of BCF and TF in H. uninervis to seasonal changes and (d) three different Kuwait sites of pollution importance to characterize H. uninervis as potential biological indicators of metal contamination.
Materials and Methods
We chose three marine sites namely, (a) Site-I (Doha), a Kuwait Bay site that is significant to industrial and domestic wastewater outfalls from power, desalination and water treatment plants, (b) Site-II (Duba’iyah), the central Kuwait coastal zone with oil wells and oil fields and (c) Site-III (Nuwaseeb), the southern most region of Kuwait observed with seldom industrialization but to certain extent of human recreational activities (Fig.1).
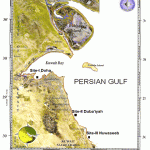 |
Figure 1: Sampling sites of Kuwait intertidal zone.
|
Seagrass, H. uninervis was collected during the years 2005-2009 from the three Kuwait marine sites on alternate months and categorized based on Kuwait’s seasons (Winter: November, January, March and Summer: May, July September). Sampling within the meadows was restricted to mono-specific areas and homogenous seagrass cover. Patches and disturbed areas were not selected for the study. From each site, a meadow with biomass (15g) was excavated from 2 x 0.25 m-2 quadrants following the earlier method6. Samples were thoroughly rinsed in seawater to remove all sediment. They were collected in sterile polyethylene labeled bags, frozen on site and transported to the lab and kept frozen at -4oC prior to analysis. The defrosted samples were scrapped with a sterile surgical blade (#11) to remove epiphytes and sections of leaves and root rhizome was rinsed in deionised water. Sub-samples were dried at 50oC to constant weight ground in Agate mortar (Reutch) and sieved in a 0.5mm Nytex mesh to fine homogenous powder.
Sediments were collected by using Van Veen Grab sampler (1000 cm2) from two quadrants adjoining the meadows samples from the three Kuwait marine sites5. Sediments were collected in sterile polyethylene bags and transported to the laboratory. Replicate sediments (20g) from each grab were dried at 50oC in an oven (GallenKamp II) until constant weight. Dried sediments were powdered in the agate mortar, homogenized and sieved in 1.0 mm sieve mesh and stored in sterile vials4, 6. Samples (0.2g) were used for trace metal analysis.
uninervis leaves, rhizome-root and sediment samples were predigested in aquaregia (Aristar grade HNO3: HCl-3:1 v/v ratio) in a polystyrene sterile centrifuge tube and left overnight. The sediment samples were treated further in 1% HF for the complete mineralization and digestion. All the samples diluted in de-ionized water (50 ml) and digested in an automatic microwave digester (Spectroprep CEM) was measured in the Analytik Jena, Zeenit-650 to determine the metals concentrations.
Trace metals bio-concentration factor (BCF) in H. uninervis was determined by calculating the ratio of metal concentration in the rhizome-roots to that of the sediment as given below:
BCF= Metal concentration in root (mg g-1) / metal concentration in sediment (mg g-1). BCF was categorized further as hyper-accumulators12 to those samples which accumulated metals above 1000 µg Kg-1. Further, the plant’s ability to translocate metals from rhizome to leaves was measured using translocation factor (TF) as given below:
TF= metal concentration in the leaves/ metal concentration in rhizomes. Wherein, TF was categorized as hyper-translocated samples that translocated metals more than 1.
Quality assurance employing replicates, standard trace metals (ICP grade), blanks and standard reference material: Orchard leaves (SRM 1571) for H. uninervis and estuarine sediment (SRM 1646A) for sediment samples from National Institute Standard Technology (NIST) assessed the precision of the instrument. Recoveries of samples (90 ±5 %) in agreement with certified values were considered as a part of quality control assessment. Statistically, Pearson’s correlation coefficient was used to correlate the significance of trace metals concentrations between the leaves, rhizome and sediment samples.
Results and Discussion
The mean trace metal concentrations was high in leaves (9.34-68.63 µg g-1and 12.32-87.89 µg g-1) followed by rhizome-root (7.35-60.13 µg g-1 and 8.35-76.13 µg g-1) and sediment (5.47-49.51 and 4.14-53.95 µg g-1 µg g-1) during winter and summer respectively (Tables 1-2). Trace metal concentrations in the present study was found higher than the earlier studies in other seagrass 1-3, 10-11, but lower trace metal concentration than the earlier studies 4,6. Trace metal concentrations in both leaves and rhizome of H. uninervis and sediment samples was observed in the sequence of Ni>Cu>Pb>Fe irrespective of the two seasons. The mean bi-monthly analysis showed trace metal concentrations in H. uninervis and sediment parts in the sequence of July > September > May > March > January > November. Furthermore, a significant relationship of increasing trace metal concentrations was observed in H. uninervis parts (leaves and rhizome) between each bimonthly samples in summer (May-September) and winter (November-March). However, the bimonthly analysis of sediment samples showed varying trace metal concentrations during the two seasons. This attributes to the mobilization of trace metals from sediment to marine flora in the marine environment and supports evidences to the earlier studies 8-9, 12. Site-wise analysis revealed H. uninervis and sediment samples collected from Site-II with high trace metal concentrations followed by Sites-I and III irrespective of the two seasons (Tables 1-2). However, the overall trace metal concentrations were observed high in summer than in winter. Further, Pearson’s statistical test revealed significant correlation coefficient between the trace metal concentrations in H. uninervis collected during summer and winter (Table 3).
Table 1: Trace metal concentrations (μg/g) in H. uninervis parts and surrounding sediment during Winter.
|
Months Sites |
Leaf Tissue | Rhizome tissue | Sediments | ||||||||||
| Cu | Pb | Ni | Fe | Cu | Pb | Ni | Fe | Cu | Pb | Ni |
Fe |
||
| Nov | Site-I | 57.06 ±1.97 | 16.89 ±1.19 | 61.91
±2.25 |
14.65 ±1.02 | 39.49 ±2.10 | 14.20 ±1.16 | 42.51 ±2.09 | 7.32 ±0.96 | 29.51 ±1.27 | 12.51 ±1.18 | 38.47 ±2.05 | 6.80 ±0.79 |
| Site-II | 74.91 ±2.59 | 23.34 ±1.24 | 92.87 ±4.25 | 15.20 ±1.13 | 50.21 ±2.13 | 22.75 ±1.19 | 91.21 ±4.22 | 7.97 ±1.02 | 49.75 ±1.97 | 20.64 ±1.29 | 56.32 ±2.15 | 7.20 ±0.89 | |
| Site-III | 34.61
±1.53 |
12.88 ±1.15 | 34.52 ±1.63 | 4.48 ±0.96 | 35.28 ±1.60 | 11.81 ±1.14 | 41.14 ±1.98 | 4.44 ±0.75 | 14.43 ±1.02 | 11.08 ±1.13 | 39.69 ±2.11 | 4.45 ±0.67 | |
| Mean | 55.53 | 17.70 | 63.10 | 11.44 | 41.66 | 16.25 | 58.29 | 6.58 | 31.23 | 14.74 | 44.83 | 6.15 | |
| Jan | Site-I | 43.46
±2.06 |
27.88 ±1.30 | 76.89 ±2.92 | 7.75 ±1.05 | 56.13 ±2.16 | 31.38 ±1.56 | 57.18 ±2.19 | 7.16 ±0.95 | 26.63 ±1.28 | 19.62 ±1.23 | 55.69 ±2.13 | 6.77 ±0.78 |
| Site-II | 73.05
±2.45 |
29.95 ±1.38 | 77.10 ±2.95 | 8.83 ±1.09 | 76.72 ±2.87 | 21.22 ±1.18 | 67.39 ±2.26 | 8.20 ±1.05 | 42.32 ±1.93 | 26.75 ±1.32 | 66.34 ±2.24 | 7.97 ±1.01 | |
| Site-III | 39.92
±1.68 |
21.12 ±1.21 | 54.29 ±2.15 | 5.01 ±0.89 | 23.03 ±1.20 | 17.83 ±1.16 | 55.36 ±2.16 | 7.13 ±0.87 | 16.28 ±1.05 | 12.60 ±1.15 | 37.54 ±1.76 | 5.38 ±0.71 | |
| 52.14 | 26.32 | 69.43 | 7.20 | 51.96 | 23.48 | 59.98 | 7.50 | 28.41 | 19.66 | 53.19 | 6.71 | ||
| Mar | Site-I | 61.80
±2.20 |
28.12 ±1.32 | 82.54 ±3.48 | 9.35 ±1.12 | 49.31 ±2.13 | 25.29 ±1.24 | 61.93 ±2.21 | 7.42 ±0.99 | 18.10 ±1.19 | 16.29 ±1.21 | 50.39 ±2.18 | 3.42 ±0.54 |
| Site-II | 79.48
±3.81 |
30.16 ±1.41 | 92.61 ±4.21 | 11.95 ±1.15 | 70.99 ±2.28 | 26.68 ±1.25 | 92.01
±4.23 |
10.01 ±1.10 | 40.10 ±1.90 | 25.44 ±1.30 | 61.85 ±2.21 | 4.17 ±0.65 | |
| Site-III | 52.10
±2.10 |
24.23 ±1.27 | 44.96 ±2.09 | 6.88 ±0.98 | 47.12 ±2.12 | 19.50 ±1.17 | 32.41 ±1.87 | 6.54 ±0.76 | 17.30 ±1.15 | 4.96 ±0.68 | 39.26 ±2.08 | 3.11 ±0.46 | |
| 64.46 | 27.50 | 73.37 | 9.39 | 55.81 | 23.82 | 62.12 | 7.99 | 25.17 | 15.56 | 50.50 | 3.57 | ||
Table 2: Trace metal concentrations (μg/g) in H. uninervis parts and surrounding sediment during Summer.
|
Months Sites |
Leaf Tissue | Rhizome tissue | Sediments | ||||||||||
| Cu | Pb | Ni | Fe | Cu | Pb | Ni | Fe | Cu | Pb | Ni |
Fe |
||
| MAY | Site-I | 72.76 ±2.61 | 28.36 ±1.15 | 97.76 ±3.86 | 17.89 ±0.95 | 54.12 ±2.60 | 16.89 ±1.12 | 62.65 ±2.78 | 8.89 ±0.80 | 26.35
±1.40 |
25.78 ±1.30 | 60.40
±2.73 |
6.85 ±0.65 |
| Site-II | 81.74 ±3.10 | 30.89
±1.25 |
99.95
±4.61 |
19.93
±1.04 |
71.04 ±2.74 | 24.32 ±1.26 | 90.85 ±4.20 | 8.93 ±0.81 | 50.92 ±2.10 | 20.25 ±1.50 | 75.85 ±2.98 | 8.51 ±0.78 | |
| Site-III | 60.18 ±2.59 | 12.87 ±1.12 | 67.53
±2.86 |
6.72
±0.91 |
61.35 ±2.60 | 12.52 ±1.09 | 57.88 ±2.69 | 6.69 ±0.68 | 23.47 ±1.23 | 11.48 ±0.91 | 38.20 ±1.61 | 4.60 ±0.52 | |
| Mean | 71.56 | 24.04 | 88.41 | 14.85 | 62.17 | 17.91 | 70.46 | 8.14 | 33.25 | 19.17 | 58.15 | 6.65 | |
| JULY | Site-I | 74.42 ±2.74 | 36.42
±1.58 |
92.04
±4.09 |
10.65 ±1.03 | 57.07 ±2.46 | 33.35 ±1.68 | 76.99 ±3.11 | 8.77 ±0.79 | 28.36 ±1.43 | 19.82 ±1.16 | 62.97 ±2.86 | 9.42 ±0.81 |
| Site-II | 92.37 ±4.19 | 46.20 ±2.06 | 105.3 ±4.76 | 12.58 ±1.10 | 79.72 ±3.13 | 38.97 ±1.72 | 88.79 ±3.98 | 10.21 ±0.87 | 43.65 ±1.98 | 29.06 ±1.45 | 75.92 ±2.98 | 8.98 ±0.79 | |
| Site-III | 68.77 ±2.78 | 35.56
±1.55 |
77.70
±3.13 |
9.62
±0.87 |
59.89 ±2.51 | 17.85 ±1.12 | 75.31
±3.08 |
7.63 ±0.74 | 25.56 ±1.29 | 15.11 ±1.10 | 41.18 ±1.86 | 7.55 ±0.75 | |
| Mean | 78.52 | 39.39 | 91.68 | 10.95 | 65.56 | 30.06 | 80.36 | 8.87 | 32.52 | 21.33 | 60.02 | 8.65 | |
| SEPT | Site-I | 74.99
±2.99 |
30.52 ±1.32 | 91.37
±3.95 |
11.59 ±1.01 | 62.31 ±2.68 | 28.05
±1.31 |
83.21 ±3.75 | 7.30 ±0.72 | 31.28 ±1.56 | 17.46 ±1.12 | 52.83 ±2.76 | 3.84 ±0.48 |
| Site-II | 85.37 ±3.96 | 31.52
±1.48 |
93.16
±4.10 |
11.98 ±1.06 | 70.45
±2.76 |
28.09
±1.35 |
92.43 ±4.70 | 9.63 ±0.84 | 41.97 ±1.95 | 25.51 ±1.27 | 67.95 ±2.97 | 4.97 ±0.69 | |
| Site-III | 69.12
±2.85 |
25.98
±1.29 |
66.24
±2.73 |
9.95
±0.98 |
59.39
±2.64 |
23.41
±1.24 |
57.08 ±2.50 | 7.21 ±0.68 | 18.51 ±1.19 | 11.22 ±0.97 | 41.06 ±1.01 | 3.82 ±0.41 | |
| Mean | 76.49 | 29.34 | 83.59 | 11.17 | 64.05 | 26.52 | 77.57 | 8.05 | 31.59 | 19.40 | 53.95 | 4.14 | |
Sites I-III: Doha, Duba’iyah, Nuwaseeb, ± values: Standard deviation
Table 3: Pearson’s correlation coefficient between trace metals in H. uninervis parts and sediment during summer and winter.
| Samples | L-W | R-W | Sd-W | L-S | R-S |
| R-W | 0.984
0.116 |
||||
| Sd-W | 0.980
0.127 |
1.000
0.011 |
|||
| L-S | 0.996
0.059 |
0.963
0.175 |
0.958
0.186 |
||
| R-S | 0.963
0.174 |
0.996
0.058 |
0.997
0.047 |
0.934
0.233 |
|
| Sd-S | 0.996
0.054 |
0.995
0.062 |
0.993
0.073 |
0.984
0.113 |
0.982
0.120 |
L: Leaf, R: rhizome, Sd: Sediment, W: winter, S: summer, Cell Contents: Pearson correlation, P-Value in italics
None of the investigated seagrass samples were classified as hyper-accumulators as they showed metal concentrations >1000µg Kg-1 in the leaves and supported the observations10. However, this species could be labeled for phytoremediation process due to high tolerance limits of this species to trace metal accumulation estimated through BCF and TF. By comparing the BCF and TF we drew conclusions on the ability of the trace metals uptake from soil to rhizome and transfer from rhizome to leaves by H. uninervis. A significant relationship existed between BCF and TF. The present study showed an increase in the BCF with decreasing TF and vice versa (Tables 4-5) and supported the earlier findings2,13. Analysis showed high TF in Site-I samples during summer (May-September) and winter (November-March) than Sites II and III. Seagrass in Kuwait Bay (Site-I) region showed high trace metals mobilization and bio-concentration when compared to trace metals in seagrass collected from the Kuwait coastal sites. The present study also indicated varying BCF and TF between summer and winter samples thus, indicating that these factors are dependent on seasonal changes in the marine environment. The overall BCF and TF were found high in summer than in winter. Statistical tests by Pearson’s correlation coefficient revealed significant BCF and TF between the seasons (summer and winter) respectively (Table 6). The overall TF in the samples were >1, indicating efficient translocation between rhizome and leaves and supported the earlier studies2, 13. The present study showed most samples with BCF and TF >1 to that of the earlier findings12, 14 indicating the potentiality for phytoextraction of trace metals. Thus, this study revealed that H. uninervis can be used as potential tool: (a) to study the pollution levels of intertidal zones, (b) to determine the interrelationship between BCF and TF to seasonal changes and (c) for phytoremediation of contaminated sites in the marine environment.
Table 4: Bioconcentration (BCF) and Translocation (TF) factors of trace metals (μg/g) to H. uninervis during winter.
| Bioconcentration factor (BCF) | Translocation Factor (TF) | ||||||||
| Cu | Pb | Ni | Fe | BCF
mean |
Pb | Ni | Fe | TF
mean |
|
| Nov | 1.33 | 1.13 | 1.10 | 1.07 | 1.44 | 1.18 | 1.45 | 2.00 | 1.52 |
| 1.00 | 1.10 | 1.61 | 1.10 | 1.49 | 1.02 | 1.01 | 1.90 | 1.36 | |
| 2.44 | 1.06 | 1.03 | 0.99 | 0.98 | 1.09 | 0.83 | 1.00 | 1.02 | |
| Jan | 2.10 | 1.59 | 1.02 | 1.05 | 0.77 | 0.88 | 1.34 | 1.08 | 1.14 |
| 1.81 | 0.79 | 1.01 | 1.02 | 0.95 | 1.41 | 1.14 | 1.07 | 1.15 | |
| 1.41 | 1.41 | 1.47 | 1.32 | 1.73 | 1.18 | 0.98 | 0.70 | 1.23 | |
| Mar | 2.72 | 1.55 | 1.23 | 2.16 | 1.25 | 1.11 | 1.33 | 1.26 | 1.12 |
| 1.77 | 1.04 | 1.48 | 2.40 | 1.11 | 1.13 | 1.06 | 1.19 | 1.16 | |
| 2.72 | 3.93 | 0.82 | 2.10 | 1.10 | 1.24 | 1.38 | 1.05 | 1.19 | |
BCF= metals in root/metals in soil, TF= metals in leaves/metals in roots, Sites I-III: Doha, Duba’iyah, Nuwaseeb
Table 5: Bioconcentration (BCF) and Translocation (TF) factors of trace metals (μg/g) to H. uninervis during summer.
|
Months Sites |
Bioconcentration factor (BCF) | Translocation Factor (TF) | |||||||||
| Cu | Pb | Ni | Fe | BCF-Mean | Cu | Pb | Ni | Fe |
TF Mean |
||
| MAY | Site-I | 2.13 | 0.65 | 1.03 | 1.28 | 1.27 | 1.34 | 1.67 | 1.56 | 2.03 | 1.65 |
| Site-II | 1.39 | 1.20 | 1.19 | 1.04 | 1.21 | 1.15 | 1.27 | 1.10 | 2.23 | 1.43 | |
| Site-III | 2.61 | 1.09 | 1.51 | 1.45 | 1.66 | 0.98 | 1.02 | 1.16 | 1.00 | 1.04 | |
| JULY | Site-I | 2.01 | 1.68 | 1.22 | 0.93 | 1.46 | 1.30 | 1.09 | 1.19 | 1.21 | 1.20 |
| Site-II | 1.82 | 1.34 | 1.16 | 1.13 | 1.36 | 1.15 | 1.18 | 1.18 | 1.23 | 1.19 | |
| Site-III | 2.34 | 1.18 | 1.82 | 1.01 | 1.59 | 1.14 | 1.99 | 1.03 | 1.26 | 1.35 | |
| SEPT | Site-I | 1.99 | 1.60 | 1.57 | 1.90 | 1.76 | 1.20 | 1.08 | 1.09 | 1.58 | 1.24 |
| Site-II | 1.67 | 1.10 | 1.36 | 1.93 | 1.51 | 1.21 | 1.12 | 1.00 | 1.24 | 1.14 | |
| Site-III | 3.20 | 2.08 | 1.39 | 1.99 | 2.16 | 1.16 | 1.10 | 1.16 | 1.38 |
1.20 |
|
BCF= metals in root/metals in soil, TF= metals in leaves/metals in roots, Sites I-III: Doha, Duba’iyah, Nuwaseeb
Table 6: Pearson’s correlation coefficient between BCF and TF during summer and winter.
| Samples | BCF-W | TF-W | BCF-S |
| TF-W | -0.180
0.643 |
||
| BCF-S | 0.922
0.001 |
-0.348
0.359 |
|
| TF-S | -0.376
0.318 |
0.915
0.001 |
-0.491
0.180 |
BCF: bioconcentration factor, TF: translocation factor, W: winter samples, S: summer
samples, Cell Contents: Pearson correlation, values in bold: significant correlation,
P-Value in italics
Acknowledgments
We express our gratitude to the Research Administration, Kuwait University for the financial support of our project (SC-01/04). We thank the faculty of Science Analytical facility (SAF), Kuwait University, for sample analysis (GS 01/01).
References
- Olsen C.R., Cutshall N.H. and Larsen I.L., Mar. Chem., 11, 501−533 (1982).
- Yoon J., Cao X., Zhou Q. and Lena Q.M., the Sci. Tot. Environ., 368,456-464 (2006).
- Butler A. and Jernakoff P., CSIRO Publisher, Melbourne (1999).
- Prange J.A. and Dennison W.C., Mar. Pollut. Bull. 41(7-12), 327-336 (2000).
- Campanella L., Conti M.E., Cubadda F. and Sucapane C. Environ. Pollut. 111(1), 117- 126 (2001).
- Conti M.E., Iacobucci M. and Cecchetti G. Internat. J. Environ. Pollut., 29 (1-3), 308-332 (2007).
- Brix H., Lyngby J.E. Estuar. Coast. Shelf Sci., 16, 455-467 (1983.
- Lanyon J., Limpus C.J. and Marsh H., Elsevier Publications, Amsterdam (1989).
- Rai L.C., Gaur J.P. and Kumar H.D Environ Res., 25, 250-259 (1981).
- Amado G.M., Creed J.C., Andrade L.R. and Pfeiffer W.C. Aquat. Bot., 80(4), 241-251 (2004).
- Lafabrie C., Pergent G., Kantin R., Pergent M.C. and Gonzalez J.L., Chemosph. 68, 2033-2039 (2007).
- Baker A.J.M. and Brooks R.R. Biorecov., 1, 81-126 (1989).
- Lewis M.A., Dantin D.D., Chancy C.A., Abel K.C. and Lewis C.G. Environ. Pollut., 146 (1), 206-218 (2007).
- Rosselli W., Keller C. and Boschi K., Plant Soil 256: 265-272 (2003.

This work is licensed under a Creative Commons Attribution 4.0 International License.





