How to Cite | Publication History | PlumX Article Matrix
S. Elumalai*, R. Kesavan1, S. Ramganesh1, V. Prakasam1 and R. Murugasen1
1Department of Plant Biology & Biotechnology, Presidency College, Chennai (India). 2SAIF (Sophisticated Analytical Instrument Facility), IIT, Chennai (India).
ABSTRACT: The bark and the leaves of Cinnamomum species are commonly used as spices and their distilled essential oils are used as flavouring agent. The extract or essential oil of Cinnamomum zeylanicum stem bark is composed of a number of compounds (Cinnamaldehyde, cinnamic acid, cinnamyl acetate, Benzyl benzoate, a-Terpineol) and not all of them appear to have antimicrobial activities. The two (C. zeylanicum and C. cassia) barks oil extracts were prepared by hydro distillation method. Streptomycin (10μg/disc) and Chloramphenicol (30μg/disc) were used as standard drug, compared with C. zeylanicum and C.cassia crude oil extract. They were used 100μl for each experiment five pathogenic bacteria Bacillus subtilis (ATCC-6633), Klebsiella pneumonia (ATCC-13883), Pseudomonas aeruginosa (ATCC-10145), Staphylococcus aureus (ATCC-126000) and Escherichia coli (ATCC-6633) were used in this study. Among all these experiments the highest percentage of growth inhibition recorded in B. subtilis (53.3%) and S.aureus (53.3%) and the lowest growth inhibition recorded in E. Coli (44.4%), K. Pneumoniae (44.4%) with C. cassia oil extracts. The highest growth inhibition recorded in E. Coli (40%) with C. zeylanicum and lowest growth inhibition recorded in S. aureus (37.8%) with C. zeylanicum. The comparative analysis of bark oil extracts of C. zeylanicum and C. cassia with all these pathogenic bacteria were studied and recorded. C. cassia showed highest growth inhibition range than the C. zeylanicum.
KEYWORDS: Cinnamaldehyde; Cinnamic acid; Benzyl benzoate; C. zeylanicum; C. cassia; Bacillus subtilis.
Download this article as:| Copy the following to cite this article: Elumalai S, Kesavan R, Ramganesh S, Prakasam V, Murugasen R. Comparative Study on Anti-Microbial Activities of Bark Oil Extract from Cinnamomum Cassia and Cinnamomum Zeylanicum. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Elumalai S, Kesavan R, Ramganesh S, Prakasam V, Murugasen R. Comparative Study on Anti-Microbial Activities of Bark Oil Extract from Cinnamomum Cassia and Cinnamomum Zeylanicum. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9321 |
Introduction
The bark and the leaves of Cinnamomum spp. are commonly used as spices in home kitchens and their distilled essential oils or synthetic analogs are used as flavouring agent in the food and beverage industry. Although traditionally known, some recent scientific studies have shown antimicrobial activity of essential oils of Cinnamomum cassia presl., C. osmophloeum kaneh. And C. zeylanicum Blume (Tiwari & Tiwari, 1997; Ferhout et al. 1999; Mastura et al. 1999; De et al., 1999; Chang et al., 2001). Quattara et al. (1997) reported the inhibitory effect of C.zeylanicum essential oil on meat deteriorating organisms. Antifungal activity was reported for respiratory tract infecting fungi such as Aspergillus niger Tieghem, A. fumigates Fres., A. nidulans (Eidam) Winter and A. flavus Link (Singh et al., 1995). Treating high moisture barley (Idler et al., 1999) with essential oil of C. zeylanicum protected them from deteriorating fungi and ochratoxin formation. Similar findings were reported for protection of stored maize (Zea mays L.) against A. flavus (Monte-Belmont & Carvajal, 1998).
The C. zeylanicum tree is endemic in India, and our initial exploratory studies have shown that hexane extract of C. zeylanicum bark protected high moisture soybean (Glycine max L) and wheat grains from storage fungi. The extract or essential oil of C. zeylanicum stem bark is composed of a number of compounds and not all of them appear to have antimicrobial activity. Therefore, for developing a reliable ready-to-use product it is necessary to identify the most active component(s) against which the final product can be standardized.
Material and Methods
Preparation of Ether Extract and Distilled Oil
Locally produced Cinnamon bark was purchased from the local spice store. All the chemical and solvents used in the study were analytical grade and distilled before use. Cinnamon bark was ground to pass through a 1-mm screen and the powder obtained was extracted at room temperature by constant percolation with ether until all the ether soluble components were removed. The solvent was evaporated using a rotator evaporator, under vacuum, at 35°C. The essential oil of the bark was obtained by hydro-distillation for 6h. The distillate was extracted twice with ether, including water soluble or dispersed components, dried over anhydrous Sodium Sulphate, and the ether was evaporated using the rotary evaporator under vacuum. The hexane extract and the distilled oil were stored in airtight screw capped vials at-10°C until used Budarri (1989), Lsman (2001).
In Vitro Antimicrobial Sensitivity Determination Test
The in vitro activities of the antibiotics by the disc diffusion method, as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) and the well diffusion test method has been used earlier days Bennet et al., 1966 after modification in 1997 Magaldi developed this procedure which named ‘well diffusion’ method. Magaldi et al., 2004 have been compared the Well diffusion method with the NCCLS broth microdillution method using several antifungal drugs.
Antimicrobial Drugs and Test Compounds
Two standard antibacterial agents (Streptomycin and Chloramphenicol) were used for the comparative studies (Table 1). The disc concentration levels are under national Committee for Clinical Laboratory Standards (NCCLS) column. The antibacterial discs were obtained from (HiMedia Laboratories Pvt. Limited, Mumbai ‘86, India.)
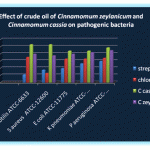
Table 1: Effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria (zone of inhibition in mm) by disc diffusion.
|
S.NO |
Organisms |
Streptomycin
(10µg/disc)
A |
Chloromphenicol
(30µg/disc)
B |
Cinnamomum cassia
(100µl) C |
Cinnamomum
Zeylanicum (100µl) D |
||||
| Inhibition | Inhibition | Inhibition | Inhibition | ||||||
| mm | (%) | mm | (%) | mm | (%) | mm | (%) | ||
| 1 | Bacillus subtilis ATCC-6633 | 10 | 11.1 | 32 | 35.6 | 48 | 53.3 | 36 | 40.0 |
| 2 | Staphylococcus aureus ATCC-12600 | 16 | 17.8 | 12 | 13.3 | 48 | 53.3 | 34 | 37.8 |
| 3 | Escherichia coli ATCC-11775 | 18 | 20.0 | 30 | 33.3 | 40 | 44.4 | 36 | 40.0 |
| 4 | Klebsiella pneumoniae ATCC-13883 | 24 | 26.7 | 30 | 33.3 | 40 | 44.4 | 38 | 42.2 |
| 5 | Pseudomonas aeruginosa ATCC-10145 | 27 | 30.0 | 31 | 34.4 | 44 | 48.9 | 36 | 45.0 |
Standard Antibiotic – Streptomycin C10 and Chloromphenicol C30 (HIMEDIA)
Preparation of Discs
The 0.5mm sterile discs were used for the test (purchase from HiMedia). The two samples of plant oil extract were loaded in the sterile disc 100µl Concentration, the discs were dried using air drier and put it in the desiccators until than experiment is carried out.
 |
Graph 1
|
Clinical Test Microorganisms
The organisms borrow from American type culture collection Two gram positive and three gram negative Staphylococcus aureus, Bacillus subtillis, Pseudomonas aeruginosa, Klebsiella pneumonia and Escherichia coli strains were used. Bacterial isolates were stored frozen in skim milk 50% glycerol at -70°C. The cultures were revived in the Muller Hinton broth or Brain heart infusion broth incubated at 35°C for 24 hours and then sub cultured on to Muller Hinton medium. All strains were recent purchased between 2006 and 2007.
Inoculum Preparation
The inoculums was prepared using gram positive and gram negative pathogens from 24 hours old cultures on Brain heart infusion agar. With a sterile loop, the tops of four to five colonies were transferred to a tube containing 5ml of Muller Hinton broth or Brain Heart infusion broth. The tube was incubated at 35°C for 24 hours. The turbidity of the culture suspension was adjusted with broth or a sterile saline solution (0.85-0.9%). The density of these cultures was adjusted with spectrophotometer at 530nm or 660nm to turbidity equivalent to that have a 0.5 McFarland standard and finally bacterial inoculums size approximately of 5 x 105 CFU/ml.
Testing Culture Media
Muller Hinton Agar (MHA) Casein acid hydrolysate: 17.5g; Beef Heart infusion: 2g; Starch soluble 1.5g; (pH 7.3 ± 0.2) Agar 17g; H2O 1000ml. Casitone agar (Bacto-casitone: 9g; yeast extract: 5g; tri-sodium citrate: 10g; glucose 20g; bactoagar; 15g; phosphate buffer: KH2PO4: 1g; Na2HPO4: 1g (pH 6.6); H2O 1000 ml). The medium was mixed well, heat to boiling water bath to dissolve the medium completely and autoclaved at 15lbs pressure (121o C) for minutes, after removal from the autoclave the sterilized medium was immediately cooled in a 50-55o C- water bath. The cooled medium was poured in to sterile Petri plates to a uniform depth of 4mm; this is equivalent to approximately 25Ml in a 90mm plate.
Antimicrobial susceptibility tests
Disk diffusion method (NCCLS M2 – A4)
The disk diffusion test (Bauer et al., 1966; Ericsson and Sherris 1971; FDA 1972; NCCLS 2002) was performed using Muller Hinton Agar (MHA) and Casitone agar. Once the medium had solidified then the culture was inoculated on the medium. Within 15minutes of adjusting the density of the inoculums, a sterile cotton swab was dipped into the standardized bacterial suspension or inoculated with 1ml of the organism suspension. The sterile swab was used to streak on the surface of the MHA medium to ensure an even distribution of the inoculums. The plates were allowed undisturbed 3-5minutes to absorption of excess moisture. The selected antibiotic disks (table) were placed on the inoculated plates and pressed firmly into the agar with the sterile forceps to ensure the complete contact with the agar. The plates were incubated at 35-37°C for 24 hours.
The percentage of inhibition was calculated by the formula,
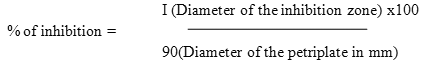
Results
The effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria (zone inhibition in mm by disc diffusion method were carried out and results were recorded.
Streptomycin (10µg/disc) and chloromphenicol (30µg/disc) were used as standard Cinnamomum zeylanicum and Cinnamomum cassia crude oil extract were used 100ml for each experiment five pathogenic bacteria Bacillus subtilis, (ATCC-6633), Staphylococcus aureus (ATCC-126000), E. Coli (ATCC-11775), Klebsiella pneumoniae (ATCC-13883), Pseudomonas aerugionsa (ATCC-10145) were used in this present study. Among all these experiments the highest percentage of growth inhibition recorded in Bacillus subtilis and S. Aureus with C. Cassia oil extracts. The lowest growth inhibition recorded in E. Coli (44.4%), K. Pneumonia (44.4%) with C. Cassia oil extracts.
The highest growth inhibition recorded in E. coli (40%) and the lowest growth inhibition recorded in S.aureus (37.8%) with C. Zeylanicum. These comparative analysis of Bark oil extracts of C. Zeylanicum and C. Cassia with all these pathogenic bacteria were recorded whereas C. cassia showed highest growth inhibition than C. zeylanicum. Further microbiological and molecular study needs to standardize the efficacy of the Bark oil extracts, with the pathogenic bacteria or cell lines, or animal model.
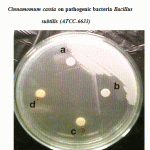 |
Figure 1: Effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria Bacillus subtilis (ATCC-6633). |
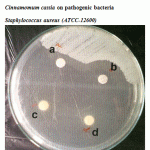 |
Figure 2: Effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria Staphylococcus aureus (ATCC-12600). |
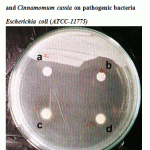 |
Figure 3: Effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria Escherichia coli (ATCC-11775). |
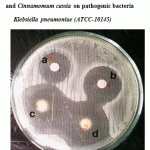 |
Figure 4: Effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria Klebsiella pneumoniae (ATCC-10145).
|
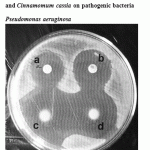 |
Figure 5: Effect of crude oil of Cinnamomum zeylanicum and Cinnamomum cassia on pathogenic bacteria Pseudomonas aeruginosa. |
Discussion
Plant essential oils have potential as natural products for organisms control because some of them are selective, often biodegrade to nontoxic products, and have little or no harmful effects on nontarget organisms (veal, 1996, Barton, Hadfield-law, 2000 and Mumcuoglu et al., 2002). They can be applied to humans in the same way as other conventional pesticides. They also provide useful information on resistance management because certain plant extracts or phytochemicals can be highly effective against insecticide-resistant insect pests (Lindquist, Adams, Hall, Adams, 1990. Laboratory and greenhouse valuations of Margosan-O against bifenthrin-resistant and –susceptible greenhouse whiteflies, Trialeurodes vaporarium (Homoptera: Aleyrodidae). Proc. the USDA neem workshop, USDA-ARS 86, Beltsville, pp. 91-99; Ahn et al.,1997). In addition, some plant essential oils or their constituents have been proposed as an alternative of the commonly used synthetic pediculicides, because they were exempted from toxicity data requirements by the US Environment Protection Agency as minimum risk pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (US EPA. 1996). Furthermore, plant essential oils are widely available and some are relatively inexpensive compared with plant extracts (Isman, 2001 ). Adulticidal activity against lice has been reported for some essential oils such as aniseed, cinnamon leaf, thyme red, tea tree, and nutmeg oils (Veal, 1996); anise and ylang ylang oils (Mumcuoglu et al., 2002); and cade, cardamone Ceylon, clove bud, eucalyptus, marjoram, myrtle, pennyroyal, rosewood, and sage oils (Yang et al., 2004). Ovicidal activity has been also reported in clove bud and leaf oils (yang et al., 2003) and Eucalyptus globules leaf oil (Yang et al., 2004). In the present study, C.zeylanicum bark essential oils exhibited potent adulticidal and ovicidal activities against P.h . capitis.
Various compounds, including phenolics, terpenoids, and alkaloids, exist in plant essential oils and jointly or independently they contribute to bioefficacy such as insecticidal, ovicidal, repellent, and antifeeding activities against various insect species (Isman, 2000 and Isman, 2001). Much effort has been focused on the determination of the distribution, nature, and practical use of plant essential oil-derived chemical substances that have insecticidal activities. Naturally occurring adulticidal and ovicidal compounds against lice include eugenol and methyl salicylate (Yang et al., 2003); and α- pinene, β- pinene, (ε) – pinocaveol, γ-terpinene, and 1- α-terpineol (Yang et al, 2004). In the current study, the pediculicidal constituents of C. zeylanicum bark essential oil were identified as benzaldehyde, (ε)- cinnamaldehyde, and linalool by GC-MS analysis. Of the compounds used, benzaldehyde, linalool, and salicylaldehyde were found to be more potent adult pediculicides than either d- phenothrin or pyrethrum. Since the louse population used appeared to be susceptible to natural pyrethrum and synthetic pyrethroids as determined by genotyping analysis of sodium channel (Yang et al, 2004 and Yang et al, 2004).A survey on Pediculus capitis (Anoplura: Pediculidae) infestation in primary school children in Korea (2002) and development of natural pediculucides for mass control. Ph.D dissertation, Chonbuk National University, Jeonju, Republic of Korea), relatively lower potency of d – phenothrin and pyrethrum was not likely due to its intrinsic resistance to the insecticides. Additionally, benzaldehyde, benzyl cinnamate, cinnamaldehyde, and salicylaldehyde were highly effective ovicides against P.h. capitis.
Elucidation of mode of action natural insecticidal products and insecticides is of practical importance for insect control because it may give useful information on the most appropriate formulation, delivery means and resistance management. Volatile compounds of many plant extracts and essential oils consist of alkanes, alcohols, aldehydes and terpeniods, particularly monoterpeniods (Coats et al., 1991). They exhibit fumigant activity against P.h. capitis. Fumigant toxicity against female P.h.capitis has been reported with eugenol and methyl salicylate (Yang et al, 2003); and 1, 8-cineole, α- pinene, β- pinene, (ε) – pinocaveol (Yang et al, 2004). In the present study, benzaldehyde, linalool, and salicylaldehyde were more effective against female P.h. capitis in closed containers than in open ones. These results indicate that the mode of delivery of the essential oil and compounds was likely by vapour action via the respiratory system, although the exact mode of action remains unknown.
Results of this indicate that C. zeylanicum bark essential oil and test compounds described could be useful as insect control fumigants or ovicides for P.h. capitis adults and eggs. Benzaldehyde (LD50 orally, 1300 mg/kg rat), Cinnamaldehyde (LD50 orally, 2220 mg/kg rat), linalool (LD50 orally, 2790 mg/kg rat), and salicylaldehyde (LD 50 orally, 520 mg/kg rat) have low acute toxicity to mammals (Budavari et al, 1989). For the practical use of Cinnnamomum bark essential oil and test compounds as novel fumigants to proceed, further research is required on the safety issues materials for human health. Other areas requiring attention are pediculicide mode of action and formulations to improve potency and stability and to reduce costs.
References
- Chalchat, J.C & Valade, I. Chemical composition of leaf oils of Cinnamomum from Madagascar: zeylanicum Blume, C. camphora L., C. fragrans Ballion and C. angustifolium. Journal of Essential Oil Research 12:537-540. 2000.
- Chang, S.T., Chen, P.F., Chang, S.C., Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. Journal of Ethanopharmacology 77, 123-127. 2001.
- Chang, S.T., Cheng, S.S., Antitermitic activity of leaf essential oil and components from Cinnamomum osmophleum Agric. Food Chem. 50, 1389-1392. 2002.
- Chang, S.T., Chen, P.P & Chang, S.C. Antimicrobial activity of leaf essential oils and their constitutents from Cinnamomum osmophloeum. Journal of Ethnopharmacology 77: 123-127. 2001.
- Channe Gowda, D and Chitra Sarathy. Strcuture of an L-arabino-D-xylan from the bark of Cinnamomum zeylanicum. Carbohydrate Research, 166, 263-269. 1987.
- Cheng, S.S., Liu, J.T., Tsai, K.H., Chen, W.J., Chang, S.T., Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum
- Cortes, H.E. Multidimensional Chromatography: Techniques and Applications, Marcel Dekker, New York, NY, pp.145-189. 1990.
- Dang, M.N., Takacsova, M., Nguyen, D.V., Kristianova, K., 2001. Antioxidant activity of essential oils from various spices. Nahrung, 45, 64-66. 2001.
- De, M., De, A.K & Banerjee A.B. Antimicrobial screening of some Indian spices. Phytotheraphy Research 13:616-618. 1999.
- Duke, J.A. Handbook of Biologically Active Phytochemicals and their Activities. CRC Press, Boca Raton, FL. 1992.
- Esau, K .1965. Plant Anatomy.
- Esau, K. 1979. Anatomy of Seed Plants.
- G., Flamini, G., Zaralli, L.J., Perrucci, S., Efficacy of an essential oil of Cinnamomum zeylanicum against Psoroptes cuniculi. Phytomedicine, 14, 227-231. 2007.
- Impar-Radosevich, J., Deas, S., Polansky, M.M., Baedke, D.A., Ingebritsen, T.S., Anderson, R.A & Graves, D.J. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: Implications for cinnamon regulation of insulin signalling. Res. 50: 177-182. 1998.
- Jayaprakash, G.K., Rao, L.J., Sakarish, K.K., Chemical composition of the flower oil of Cinnamomum zeylanicum Journal of Agricultural & Food Chemistry 48, 4294-4295.
- Jayaprakash, G.K., Negi, P.S., Jena, B.S., Jaganmohan Rao, L., Antioxidant and antimutagenic activities of Cinnmomum zeylanicum fruit extracts. Journal of Food Composition and Analysis 20, 330-336. 2007.
- Jayaprakash, G.K., Rao, L.J & Sakariah, K.K. Chemical composition of volatile oil from Cinnamomum zelanicum Z. Naturforsch 57:990993. 2002.
- Jirovetz, L, Buchbauer, G. Ngassoum, M.B & Eberhardt, R. Ananlysis and quality control of the essential oil of the leaves of Cinnamomum zeylanicum L. From Cameroon. Ernahrung 22: 443-445. 1998.
- Johansen, DA. 1940. Plant Microtechnique.
- Kitazuru, E. R., Moreira, A.V.B., Mancini-Filho, J., Delincee, H., Villavicencio, A.L.C.H., Effects of irradiation on natural antioxidants of cinnamon (Cinnamomum zeylanicum N). Radiation and Chemistry 71, 37-39. 2004.
- Lee, H.S. & Ahn, Yj. Growth – Inhibiting effects of Cinnamomum cassia bark-derived materials on human intestinal bacteria. Agric. Food Chem 46: 8-12. 1998.
- Mancini-Filho, J., Van-Koiij, A., Cozzolino, F.F & Torres, R.P. Antioxidant activity of cinnamon (Cinnamomum zeylanicum, Breyne) extracts. Chim. Farm. 137: 443-447. 1998.
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry, Wiley, New York, pp. 193-199. 1976.
- M., Azah, M., Khozirah, S., Mawardi, R. & Manaf, A.A. Anticandidal and antidermatophytic activity of Cinnamomum species essential oils Cytobios 98: 17-23. 1999.
- Nath, S.C., Pathak, M.G & Baruah, A. Benzyl benzoate, the major component of the leaf and stem bark oil of Cinnamomum zeylanicum Journal of Essential Oil Research 8:327-328. 1996.
- Raina, V.K., Srivastava, S.K., Aggrawal, K.K., Ramesh, S., Kumar, Shushil. Essential oil composition of Cinnamomum zeylanicum Blume leaves form little andamn, India. Flavour Fragrance Journal 16, 374. 2001.
- Ross, M.S.F. Analysis of cinnamon oils by high-pressure liquid chromatography. Journal of Chromatography 118:273-275. 1975
- P. & Bicchi, C. Capillary Gas Chromatography in Essential Oil Analysis, Huthigh, Heidelberg, 1987.

This work is licensed under a Creative Commons Attribution 4.0 International License.





