How to Cite | Publication History | PlumX Article Matrix
P. Iyapparaj*, G. Immanuel, R. Ramasubburayan, P. Esakkiraj, Sankaralingam, M. Navin Chandran and A. Palavesam
Centre for Marine Science and Technology, Manonmaniam Sundaranar University, Rajakkamangalam - 629 502 (India).
ABSTRACT: Effect of different wavebands of ultraviolet radiation (UV–A and UV–B) on pigments profile of south Indian macroalgae Gracillaria edulis and Hypnea musciformis was assessed under invitro conditions. In general, the Chlorophyll – a (Chl a) was higher than the Chlorophyll – b (Chl b) content in the candidate seaweeds. The long wavelength UV–A progressively increased Chl a, Chl b and total chlorophyll content correspond to the increase in exposure duration in both the seaweeds. But the short wavelength UV–B considerably reduced the chlorophyll pigments. The total carotenoid content of the selected seaweed species exposed to UV–A and UV–B irradiation was found to be increased notably as a measure of photo protective function to guard the photosynthetic apparatus from damage and to resist the photo oxidation.
KEYWORDS: UV-A; UV–B; Chlorophyll; Carotenoid; Macroalgae;
Download this article as:| Copy the following to cite this article: Iyapparaj P, Immanuel G, Ramasubburayan R, Esakkiraj P, Sankaralingam, Chandran M. N, Palavesam A.. Effect of Ultra Violet Radiation on Pigments Profile of Seaweeds Gracillaria Edulis and Hypnea Musciformis.Biosci Biotechnol Res Asia 2010;7(1) |
| Copy the following to cite this URL: Iyapparaj P, Immanuel G, Ramasubburayan R, Esakkiraj P, Sankaralingam, Chandran M. N, Palavesam A.. Effect of Ultra Violet Radiation on Pigments Profile of Seaweeds Gracillaria Edulis and Hypnea Musciformis.Biosci Biotechnol Res Asia 2010;7(1).Available from: https://www.biotech-asia.org/?p=9241 |
Introduction
Seaweeds are the sedentary plant biota of sea, one of the major contributing communities in marine primary producers. Especially, algae are adapted well to survive in intertidal zone where as it has to face the drastic change in the incident solar radiation and variation in climate to the greater extent because algae are uncovered during low tide or floating near the water surface. The above variation in physiological parameters leads to strong photo inhibition in algae (Hanelt, 1992; Henley et al., 1992; and Hanelt et al., 1994a). Intertidal zone is potentially vulnerable zone to photo inhibition or damage by photosynthetically active radiation or UV radiation (Wood, 1987). Mainly the ecology of seaweed is attributed with their ability to absorb and efficiently manage the incident radiant energy (Luning, 1990).
The stratospheric depletion of ozone layer results in increased levels of incident ultraviolet radiation on the crust and aquatic bodies also not excluded to this. Despite of more anthropogenic and natural destruction is leading to larger depletion in ozone layer results in enhanced UV-B radiation on earth. Both UV-A (315-400 nm) and UV-B (280-320 nm) radiation are capable of penetrating the water column to an ecologically significant depth (Calkins and Thordardottir, 1982; Smith et al., 1992). On comparison with other organism, the effects of UV radiation on plants are huge (Bornman and Teramura, 1993; Holm-Hansen et.al., 1993). The intertidal algae may possess photo adaptive mechanism to minimize the damage by solar UV and plants of sub tidal zone are more sensitive than the plant species of intertidal zone (Polne and Gibor, 1982).
Still now, conflicting reports arise about the regulatory effects of UV-A radiation. Hashimoto and Tajima (1980) and Biswal et al., (1997) found inhibition of total chlorophyll and carotenoid contents induced by UV-A. Promotory effects of UV-A on the synthesis of Chl and carotenoids were also reported by Senger and Schmidt (1986) and Rau and Schrott (1984). Photo repair and photo reactivation processes may be stimulated by radiation in the blue and UV-A spectral regions which activate photolyase (Sutherland 1981, Pang and Hays, 1991).
In total solar energy, 1.5% was constituted by UV-B radiation and showed its impacts on biological systems (Teramura et al.,1980). Photosynthesis (Bischof et al., 1999; Brouwer et al., 2000) and growth (Aguilera et al., 1999; Altamirano et al., 2000) reflected due to the acclimation of seaweeds to UV-B exposure to a certain extent. Physiologically sensitive indicators can recognize UV-B related stress at an early stage (Cordi et al., 1997, 1999). So far, only scanty data have been collected on effects of UV radiation on macro algae from in situ experiment and few field studies have been done in Arctic region (Hanelt et al., 1997a; Aguilera et al., 1999; Brouwer et al., 2000 and Bischof et al., 2001). In the light of the above, the present work has been carried out to investigate the impact of UV irradiation on pigments profile of marine macroalage G. edulis and H. musciformis.
Materials and Methods
Experimental samples
Marine macroalgae G. edulis and H. musciformis were collected from the rocky shore with the depth about 1.0 to 1.5 metre at Leepuram, Kanyakumari District, Tamilnadu, India (Lat. 8006’ 46.1” and Long. 77033’ 21.9”). Immediately after collection, they were brought to the laboratory and were washed thoroughly in sterile seawater to remove the adherent particles and debris etc. Then the seaweeds were kept individually in the enriched seawater medium of Provasoli (1968) for further study.
Experimental setup
The seaweeds in the seawater enriched medium are divided into three groups, one control group and two experimental groups. The control group is kept in normal light of 1000 lux and the experimental groups were subjected to UV–A (320 – 400nm) and UV–B (280 – 320nm) treatments respectively using the UV chamber (ADVANCE, SLW 6W, India) for a period of 8h. The experimental samples were collected at an interval of every 2h.
Estimation of chlorophyll
Determination of chlorophyll a, b and Total chlorophyll.
The amount of chlorophyll contents of the seaweeds were estimated by the method of Arnon (1949). 500 mg of experimental sample was kept in a pestle and mortar with 10 ml of 80% acetone and it was ground well. The homogenate was centrifuged at 500 x g for 15 minutes and the supernatant was stored. The pellet was re-extracted by repeated washing with 5 ml of 80 % acetone till it become colourless. All the extracts were pooled and utilized for chlorophyll determination and the absorbance was measured at 645 nm and 663 nm in a UV visible spectrophotometer (Techomp 8500, Hongkong).
Estimation of total carotenoids
For carotenoid extraction, a gram of experimental samples were macerated individually with acetone : water (9 : 1). The obtained homogenate was centrifuged at 500 x g for 20 minutes in order to obtain clear supernatant. The extracted carotenoid sample was diluted to appropriate volume so as to obtain the optical density value of 0.8 or less. For that, the same solvent system used for the carotenoid extraction was used. After proper dilution, the sample was centrifuged and the clear supernatant obtained was used to measure carotenoid by taking optical density at 444 nm (Rodriguez-Amaya , 1993).
Absorption spectra (λ max)
The carotenoid samples extracted from the respective experimental samples were also used for absorption spectral analysis. The samples were scanned from 350 nm to 500 nm in a UV-visible Spectrophotometer by follwing the methods of Rodriguez-Amaya (1993).
Statistical analysis
The experiment and control set were repeated at least three times for each pigment analysis and the results were compared to the control through the one way and two way analysis of variance described by Zar (1974).
Results and Discussion
In the present study, the effect of UV-irradiation on major photosynthetic pigments such as Chl a, b, total chlorophyll content, carotenoids content and its profile of marine macroalgae G. edulis and H. musciformis were investigated. The results revealed that, the short wave length UV–B was more adverse than the long wavelength UV–A radiation. Usually UV–A radiation exhibits both positive and negative effects on plant photosynthesis (Wellmann, 1983); whereas, UV–A radiation activates gene expression for photosystem (PS II), reaction centre proteins (Christopher and Mullet, 1994) and also it inflicts damage to photosynthetic apparatus (Joshi et al., 1997; Turcsanyi and Vass, 2000). Yet today, conflicting reports were raised about the regulatory effects of UV–A radiation. Hashimoto and Tajima (1980) and Biswal et al., (1997) found inhibition of total chlorophyll and carotenoids content induced by UV–A radiation. The promoting effects of UV–A radiation on the synthesis of chlorophyll and carotenoids were also reported by Senger and Scmidt (1986) and Rau and Schrott (1984).
In the present study, G. edulis during UV–A exposure, chlorophyll a and b contents were remarkably enhanced and reached maximum at the end of the experiment (8th h). The increase in UV–A exposure duration subsequently increased the chlorophyll content over the control. In general, the Chl a content was higher than the Chl b content. The Chl a content increased form 1.25 ± 0.020 to 2.71 ± 0.061µg/g. Chl b content also enhanced from 0.32 ± 0.016 to 1.47 ± 0.045 µg/g. Like wise the total chlorophyll content also enhanced from 1.77 ± 0.033 to 5.45 ± 0.033 µg/g (Table 1). Influence of UV–A exposure duration was statistically significant (p<0.05) for Chl b and it was not statistically significant for Chl a (p>0.05) where as it was statistically more significant (p< 0.0001) for total chlorophyll content.
In H. musciformis also enhanced chlorophyll pigments were noticed during UV–A exposure. The exposure duration, increased the chlorophyll pigments (Chl a, b and total chlorophyll content). Chl a content increased from 2.83 ± 0.045 to 4.15 ± 0.061 µg/g and also Chl b is enhanced from 0.94 ± 0.032 to 2.02 ± 0.016 µg/g. Similarly, total chlorophyll content also increased from 3.94 ± 0.008 to 6.19 ± 0.028 µg/g (Table 2). The influence of UV–A exposure duration on Chl a and Chl b content were not statistically significant (p> 0.05) and for total chlorophyll content it was statistically more significant (p< 0.0001). For both G. edulis and H. musciformis, chlorophyll (a, b and total chlorophyll) content registered an increasing trend. Similar result was also reported by Dohler (1998).
UV–B radiation known to affect a wide range of functional aspects including gene variations (Jordan et al., 1996), biochemical and physiological changes (Eswaran et al., 1993), behaviour and ecological (Behrenfeld et al., 1994) systems in photosynthetic organisms. Effect of UV–B radiation in marine plants reported to affect the PS II activity (Worrest, 1983), DNA damage (Karentz et al., 1991a) and inhibition of Rubisco activity (Lassar et al.,, 1994). Changes in the levels of photosynthetic pigments in (Kappaphycus) seaweed exposed to UV–B radiation was also investigated by Eswaran and Subba Rao (2001).
In G.edulis, the Chl a, b and total chlorophyll content declined during UV–B exposure and it showed a significant linear trend with exposure duration. The Chl a content significantly (p<0.05) decreased from 1.01 ± 0.016 to 0.54 to 0.012 µg/g. Likewise, the Chl b content also significantly (p<0.05) decreased from 0.43 ± 0.020 to 0.16 ± 0.044 µg/g at sixth hour of exposure and during 8th hour, no Chl b content was assessed. The total chlorophyll content also expressed a declining trend from 1.69 ± 0.020 to 0.63 ± 0.012 µg/g (Table 1) which was statistically more significantly (p< 0.0001).
In H. musciformis, the UV–B irradiation reduced the Chl a content from 2.53 ± 0.069 µg/g to 1.32 to 0.045 µg/g and the Chl b content from 0.86 ± 0.032 µg/g to 0.11 to 0.008 µg/g at the end of the experiment. Total chlorophyll content was also decreased remarkably with subsequent increase in UV–B exposure duration from 3.53 ± 0.016 to 1.47 ± 0.020 µg/g (Table 2). Influence of UV–B exposure duration on Chl b was statistically significant (p<0.05) and for chlorophyll a it was not significant (p<0.05) where as the decrease in total chlorophyll content was statistically more significant (p< 0.0001).
Similarly, photosynthetic pigments have been shown to represent critical targets of UV–B radiation (Vass, 1997). Ambient levels of UV–B radiation have already been demonstrated to be effective in reducing the concentration of all major photosynthetic pigments in natural populations of Antartic phytoplankton (Bidigare, 1989) as well as in different macroalgae from the North Sea (Dohler et al., 1995). Further, the report of Lingakumar and Kulandaivelu (1998) was in agreement with the UV–B induced decrease in chlorophyll pigments.
The total carotenoid content of G. edulis established an increasing trend for both UV–A and UV–B radiation. The increase was from 0.0076 ± 0.00030 µg/g to 0.0097 ± 0.00033 µg/g in UV–A and from 0.0074 ± 0.00031 to 0.0091 ± 0.00027 µg/g for UV–B exposure respectively over the control value of 0.0073 ± 0.00029 µg/g. Variation in carotenoid content due to exposure duration was statistically significant (P < 0.001), similarly the variation in carotenoid content due to UV–A and UV–B radiation was also statistically significant (P < 0.05) (Fig.1).
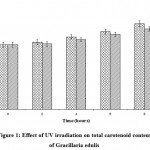 |
Figure1: Effect of UV irradiation on total carotenoid content of Gracillaria edulis.
|
In H. musciformis also the carotenoid content enhanced from 0.0056 ± 0.00048 µg/g to 0.0089 ± 0.00030 in UV–A and from 0.0053 ± 0.00068 to 0.0085 ± 0.00029 µg/g in UV–B exposure respectively against the control value of 0.0052 ± 0.00033 µg/g. Variation in carotenoid content due to exposure duration was statistically significant (P < 0.0001), similarly the variation in carotenoid content due to UV–A and UV–B radiation was also statistically significant (P < 0.05) (Fig.2).
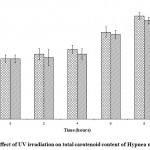 |
Figure 2: Effect of UV irradiation on total carotenoid content of Hypnea musciformis.
|
Carotenoids are the accessory photosynthetic pigments (Young, 1991; Yamamoto and Bassi, 1996) present in plants ubiquitously which responds well against the UV radiation to guard the photosynthetic apparatus from damage and resist the photooxidation (Demmig – Adams, 1990; Gilmore, 1997). The observed increase in carotenoid contents was attributed to the protection of photosynthetic apparatus.
The reactivity of carotenoid profile to UV irradiation is species dependent. During spectral analysis, in G. edulis both the UV – A and UV–B radiations did not exert any conflicts in carotenoid profile in all the exposures and control. The absorption maxima (λ max) at 430nm confirmed the presence of Zeaxanthin. On the other hand H. musciformis was highly reactive with UV exposure and during UV–A exposure, expression of unknown carotenoids, γ-carotene and zeaxanthin were noticed but the control sample concluded the presence of violaxanthin and during UV–B exposure, expression of unknown carotenoids, zeaxanthin and neoxanthin were recorded against the control profile of unknown carotenoid and zeaxanthin (Table. 3)
Table 1: Effect of UV irradiation on chlorophyll content of Gracillaria edulis
| Conditions | Duration of exposure (h) | Chlorophyll pigments (µg/g) | Total Chlorophyll (µg/g) | |
a |
b |
|||
| Control | 0 | 1.25 ± 0.020 | 0.32 ± 0.016 | 1.77 ± 0.033 |
| UV-A | 2 | 1.63 ± 0.012 | 0.75 ± 0.028 | 2.70 ± 0.020 |
| 4 | 1.97 ± 0.033 | 0.91 ± 0.012 | 3.42 ± 0.045 | |
| 6 | 2.15 ± 0.044 | 1.18 ± 0.033 | 4.19 ± 0.012 | |
| 8 | 2.71 ± 0.061 | 1.47 ± 0.045 | 5.45 ± 0.033 | |
| UV-B | 2 | 1.01 ± 0.016 | 0.43± 0.020 | 1.69 ± 0.020 |
| 4 | 0.96 ± 0.028 | 0.27 ± 0.028 | 1.37 ± 0.020 | |
| 6 | 0.72 ± 0.008 | 0.16 ± 0.044 | 0.97 ± 0.028 | |
| 8 | 0.54 ± 0.012 | ND | 0.63 ± 0.012 | |
ND : Not Detected ; Each value is a mean of triplicates
Table 2: Effect of UV irradiation on chlorophyll content of Hypnea musciformis.
| Conditions | Duration of exposure (h) | Chlorophyll pigments (µg/g) | Total Chlorophyll (µg/g) | |
| a | b | |||
| Control | 0 | 2.83 ± 0.045 | 0.94 ± 0.032 | 3.94 ± 0.008 |
| UV-A | 2 | 3.06 ± 0.044 | 1.20 ± 0.037 | 4.53 ± 0.020 |
| 4 | 3.68 ± 0.020 | 1.56 ± 0.028 | 5.57 ± 0.016 | |
| 6 | 3.91 ± 0.033 | 1.79 ± 0.020 | 6.19 ± 0.028 | |
| 8 | 4.15 ± 0.061 | 2.02 ± 0.016 | 6.78 ± 0.044 | |
| UV-B | 2 | 2.53 ± 0.069 | 0.86± 0.032 | 3.53± 0.016 |
| 4 | 2.08 ± 0.020 | 0.52 ± 0.028 | 2.83 ± 0.020 | |
| 6 | 1.74 ± 0.033 | 0.33 ± 0.020 | 2.28 ± 0.016 | |
| 8 | 1.32 ± 0.045 | 0.11 ± 0.008 | 1.47 ± 0.020 | |
Each value is a mean of triplicates
Table 3: Effect of UV irradiation on carotenoid profile of Gracillaria edulis and Hypnea musciformis
| Conditions | Duration of exposure (h) | Gracillaria edulis | Hypnea musciformis | ||
| λ max | Name of the carotenoids | λ max | Name of the carotenoids | ||
| Control | 0 | 430 | Zeaxanthin | 370
442 |
Unknown
Violaxathin |
| UV-A | 2 | 430 | Zeaxanthin | 370
439 |
Unknown
g-carotene |
| 4 | 430 | Zeaxanthin | 370
439 |
Unknown
g-carotene |
|
| 6 | 430 | Zeaxanthin | 411
370 |
Unknown
Unknown |
|
| 8 | 430 | Zeaxanthin | 370
430 |
Unknown
Zeaxanthin |
|
| UV-B | 2 | 430 | Zeaxanthin | 370
430 |
Unknown
Zeaxanthin |
| 4 | 430 | Zeaxanthin | 470
430 |
Neoxanthin
Zeaxanthin |
|
| 6 | 430 | Zeaxanthin | 430
470 |
Zeaxanthin
Neoxanthin |
|
| 8 | 430 | Zeaxanthin | 370
470 |
Unknown
Zeaxanthin |
|
Protection of photoxydative damage aids by dissipating excessively absorbed light energy in addition to xanthophylls cycle (Schafer et al., 1994; Niyogi et al., 1997). The general contribution of xanthophylls cycle to the protection of marine macroalgae from photodamage by high levels of PAR has previously been demonstrated by several authors such are Vershinin and Kamnev (1996), Hanelt et al., (1997b) and Schofield et al., (1998). Xanthophylls are the carotenoids, accessory pigments of seaweeds. Hence the change in carotenoid profile was in accordance with the earlier findings regarding the photo guarding activity of marine macroalgae.
Conclusion
Seaweeds are one of the important marine eco systems responsible for the productivity in marine food web. Adverse impact of UV radiation on seaweed community disturbs the major bio portions of the ocean. The present study revealed that UV–B was more deleterious than UV–A in concern with the photosynthetic and accessory pigments of seaweeds. Hence it’s hourly need to channelize further studies in this aspect to protect the seaweed community from the harmful effects of UV radiation.
References
- Hanelt, D., 1992. Photoinhibition of photosynthesis in marine macrophytes of the South Chinese Sea, Mar. Ecol. Prog Ser., 82: 199-206.
- Henley, W.J., Lindley S.T., Levavasseur G et al., 1992. Photosynthetic response of Ufua rotunduta to light and temperature during emersion on an intertidal sand flat, Qecologia, 89: 516-523.
- Hanelt, D., Jaramillo M.J., Nultsch W et al., 1994a. Photoinhibition as a regulative mechanism of photosynthesis in marine algae of Antarctica, Ser. Cient. INACH, 44: 67-77.
- Wood, W.F., 1987. Effect of solar ultraviolet radiation on the kelp Ecklonia radiata, Mar. Biol., 96: 143-150.
- Luning, K., 1990. Seaweed. Their Environment, Biogeography and Ecophysiology, John Wiley & Sons, New York, p. 527.
- Calkins, J. and Thordardottir T, 1982. Penetration of solar UV-B into waters of Iceland. In: Calkins, J. (Ed.), The Role of Solar Ultraviolet Radiation in Marine Ecosystems. Plenum, New York, pp. 309–321.
- Smith, R.C., Prezelin B.B, Baker K.S et al., 1992. Ozone depletion: ultraviolet radiation and phytoplancton biology in Antarctic waters. Science., 255: 952–959.
- Bornman, J.F. and Teramura A.H, 1993. Effects of ultraviolet-B radiation on Terrestrial plants. in A.R. Young, L.O. Bjsm, J. Moan and W. Nultsch (eds.), Environmental UVPhotobiology, Plenum, New York, London, 427-471.
- Holm-Hansen, O., Lubin D. and Helbling E.W, 1993. Ultraviolet radiation and its effects on organisms in aquatic environments, in A.R. Young, L.O. Bjsm, J. Moan and W. Nultsch (eds.). Environmental UV Photobiology, Plenum, New York, London, 379-425.
- Polne, M. and Gibor A, 1982. The effect of high intensity UV radiation on benthic marine algae, in J. Calkins (ed.), The Role of Solar Ultraviolet Radiation in Marine Ecosystems, Plenum, New York, 573-579.
- Hashimoto, T. and Tajima M, 1980. Effects of ultra-violet irradiation on growth and pigmentation in seedlings. Plant Cell Physiol., 21 : 1559 – 1571.
- Biswal, B., Joshi P.N. and Kulandaivelu G, 1997. Changes in leaf protein and pigment contents and photosynthetic activities during senescence of detached maize leaves: influence of different ultraviolet radiations. Photosynthetica, 34: 37 – 44.
- Senger, H. and Schmidt W, 1986. Diversity of photoreceptors. In : Kendrick, R. E., Kronenberg, G. H. M. (Ed.) : Photomorphogenesis of plants. Martinez, Nijhoff / Dr, W. Junk Publ. Dondrect-Boston, Lancaster, 137 – 158.
- Rau, W. and Schrott E.L, 1984. Blue light control as pigment biosynthesis. In : Senger, H. (Ed.) : Blue light responses phenomena and occurrence in plants and microorganisms. CRC Press, Boca Raton, 1: 43 – 64.
- Sutherland, B.M., 1981. Photoreactivation – Bioscience. 31 : 439 -444.
- Pang, Q. and Hays J.B, 1991. UV–B inducible and temperature – sensitive photoreactivation of cyclobutane pyrimidine dimers in Arabidopsis thaliana. Plant Physiol. 65: 536 -543.
- Teramura, A.H., Biggs R.H. and Kossuth S, 1980. Effects of ultraviolet – B irradiances on soybean. II. Interaction between ultraviolet – B and photosynthetically active radiation on net photosynthesis, dark respiration, and transpiration. Plant Physiol. 65. 483 – 488.
- Bischof, K., Hanelt D. and Wiencke C, 1999. Acclimation of maximal quantum yield of photosynthesis in the brown alga Alaria esculenta under high light and UV radiation. Plant Biol 1 : 435 – 444.
- Brouwer, P.E.M., Bischof K, Hanelt D et al., 2000. Photosynthesis of two Arctic macroalgae under different ambient radiation levels and their sensitivity to enhanced UV radiation. Polar Biol 23 : 257 – 264.
- Aguilera, J., Karsten U, Lippert H et al., 1999. Effects of solar radiation on growth, photosynthesis and respiration of marine macro algae from the Arctic. Mar Ecol Prog Ser 191:109 – 119.
- Altamirano, M., Flores-Moya A. and Figueroa F.L, 2000. Long-term effects of natural sunlight under various ultraviolet radiation conditions on growth and photosynthesis of intertidal Ulva rigida (Chlorophyceae) cultivated in situ. Bot Mar 43 : 19 – 126.
- Cordi, B., Depledge M.H, Price D.N et al., 1997. Evaluation of chlorophyll fluorescence, in vivo spectro photometric pigment absorption and ion leakage as biomarkers of UV–B exposure in marine macroalgae. Mar Biol., 130 : 41 – 49.
- Cordi, B., Depledge M.H, Price D.N et al., 1999. Evaluation of in vivo thallus absorptance and chlorophyll fluorescence as biomarkers of UV–B exposure and effects in marine macroalgae from different tidal levels. Mar Environ Res., 48 : 193 – 212.
- Hanelt, D., Wiencke C. and Nultsch W, 1997a. Influence of UV radiation on the photosynthesis of Arctic macroalgae in the field. J Photochem Photobiol, 38 : 40–47
- Bischof, K., Hanelt D. and Wiencke C, 2001. UV-radiation and Arctic marine macroalgae. In: Hessen D (ed) UV-radiation and Arctic ecosystems. Ecological studies series, Springer, Berlin Heidelberg New York, 153 : 227 – 243.
- Provasoli, L., 1968. Media and prospects for the cultivation of marine algae. In: Watanable A, Hattori A, eds. Cultures and collections of algae. Proceedings of the US, Japan Conference, Hakone. Japanese Society of Plant Physiology, 63-75.
- Arnon, D.I., 1949. Copper enzymes in isolated chloroplast, polyphenol oxidase in Beta vulgarise. Plant Physiol., 2: 1-15.
- Rodriguez-Amaya, D.B., 1993. Nature and distribution of carotenoids in foods. In Charalambous G (ed), Shelf life studies of foods and beverages. Chemical, biological, physical and nutritional aspects. Elsevier Science Publishers, Amsterdam, 547-589.
- Zar, J.E., 1974. Biostatistical analysis. Prentice Hall, New Jersy, p. 620.
- Wellmann, E., 1983. UV radiation in photomorphogenesis. In : Shropshire, W., Jr., Mohr, H. (Ed.) : Photomorphogenesis, Springer : Berlin, 745 – 756.
- Christopher, D.A. and Mullet J.E, 1994. Separate photosensory pathways co-regular blue light ultraviolet-A-activated PSB-D-Psbc transcription and light – induced D2 and CP43 degradation in Barley (Hordeum vulgare) chloroplasts. Plant Physiol., 104: 1119 – 1129.
- Joshi, P.N., Ramaswamy N.K, Raval M.K et al., 1997. Response or sensory levels of wheat seedlings to UV–A radiation : inhibition of PSII activity in light and darkness. Environ. Exp. Bot, 38: 237 – 242.
- Turcsanyi, E. and Vass I, 2000. Inhibition of photosynthetic electron transport by UV–A radiation targets the photosystem II complex. Photochem. Photobiol., 72 : 513 – 520.
- Dohler, G., 1998. Effects of UV-radiation on pigments of the Antarctic macroalgae Leptosomia simplex L. Photosynthetica, 35(3) : 473 – 476.
- Jordan, B.R., 1996. The effects of UV–B radiation on plants: A molecular perspective. Adv. bot. Res. 15. 91 – 98.
- Eswaran, K., Parankumar A. and Kulandaivelu G, 1993. Impact of enhancement UV–B on photosynthetic and biochemical characteristics of maize under water stress. Plant Physiol. Biochem., 20 : 36 – 40.
- Beherenfeld, M.J., Tee H. and Small L.F, 1994. Interaction between nutritional status and long term responses to UV–B radiation stress in marine diatom. Mol. Biol., 523 – 530.
- Worrest, R.C., 1983. Impact of solar ultraviolet-B radiation (290-320 nm) upon marine macroalgae. Physiol. Plant, 58 : 428 – 434.
- Karentz, D., Cleaver J.E. and Mitchell D.L, 1991a. Cell survival characteristics and molecular response of Antarctic phytoplankton to UV–B radiation. J. Phycology, 27: 326 – 341.
- Lassar, M.P., Cullen J.J. and Neale B.J, 1994 Carbon uptake in a marine diatom during acute exposure of UV–B radiation : relative importance of damage and repair. J. Phycology, 30: 183 – 192.
- Eswaran, K. and Subbarao P.V, 2001. Impact of UV–B radiation on a marine red alga Kappaphycus alvarezii (Soberiaceae, Rhodophyta). Indian J. Mar. Sci., 30: 105 – 107.
- Vass, I., 1997. Adverse effects of UV–B light on the structure and function of the photosynthetic apparatus. In: Pessarakali M (ed) Handbook of photosynthesis. Dekker, New York, pp 931 -949.
- Bidigare, P.R., 1989. Potential effects of UV–B radiation on marine organisms of the Southern Ocean: distributions of phytoplankton and krill during austral spring. Photochem Photobiol 50: 469 – 477.
- Dohler, G., Hagmeier E. and David C, 1995. Effects of solar and artificial UV irradiance on pigments and assimilation of 15N ammonium and 15N nitrate by macroalgae. J. Photochem. Photobiol., 30: 179 – 187.
- Lingakumar, K. and Kulandaivelu G, 1998 Differential responses of growth and photosynthesis in Cyampsis tetragonoloba L. brown under ultraviolet-B and supplemental long wavelength radiations. Photosynthetica 35 (3): 335 – 343.
- Young, A.J., 1991. The photoprotective role of carotenoids in higher plants. Physiol. Plant., 83 : 702 – 708.
- Yamamoto, H.Y. and Bassi R, 1996. Carotenoids: localization and function. In : Ort, D. R., Yocom, C. F. (Ed.) : Oxygenic photosynthesis. The light reaction, Klewer Academic Publishers, Dordrect, Boston, London, 539 – 563.
- Demmig – Adams, B., 1990. Carotenoids and photoprotection in plants: A role for Xanthophyll zeaxanthin. Reviews on Bioenergetics, Biochemica and Biophysica Acta., 1020: 1 – 24.
- Gilmore, A.M., 1997. Mechanistic aspects of xanthophylls cycle dependent photo protection in higher plants chloroplasts and leaves. Physiol. Plant, 99: 197 – 209.
- Schafer, C., Schmidt V. and Roos M, 1994. Characterization of high light – induced increases in xanthophyll cycle pigment and lutein contents in photoautotrophic cell cultures. J. Photochem. Photobiol. B: Biol. 22: 67 –75.
- Niyogi, K.K., Bjorkman O. and Grossman A.R, 1997. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci., USA. 94: 14162 – 14167.
- Vershinin, A.O. and Kamnev A.N, 1996. Xanthophyll cycle in marine macroalgae. Bot. Mar. 39: 421 – 425.
- Hanelt, D., Wiencke C., Kartsen U et al., 1997b. Photo inhibition and recovery after high light stress in different developmental and life-history stages of Laminaria saccharina (Phaeophyta). J. Phycol., 33 : 387 – 395.
- Schofield, O., Evens T.J. and Millie D.F, 1998. Photosystem II quantum yields and xanthophyll – cycle pigments of the macroalgae Sargassum natans (Phaeophyceae) : responses under natural sunlight. J.Phycol. 34: 104 – 112.

This work is licensed under a Creative Commons Attribution 4.0 International License.





