How to Cite | Publication History | PlumX Article Matrix
Talat, K*. and Anwar, S. Y.
Department of Genetics, Osmania University, Hyderabad, 500 007 A.P. INDIA. Corresponding Author e.mail: talatkh@yahoo.co.in
ABSTRACT: Present study describes in detail induction of somatic embryogenesis and plantlet regeneration through somatic embryos in different genotypes of safflower. Cotyledons, hypocotyls and root explants of ten different genotypes of safflower (Carthamus tinctorius L) were inoculated on to two different media MS and B5 mainly differing in their inorganic and organic constituents and plant growth regulators to study their influence on the induction of somatic embryogenesis and plantlet regeneration. Among the two different media, B5 medium supplemented with 2,4,5- Trichlorophenoxy acetic acid (2,4,5-T) showed relatively low frequency of somatic embryogenesis while Murashige and Skoog (MS) medium supplemented with same auxin favored maximum frequency of somatic embryogenesis, instead of 2,4-dichlorophenoxy acetic acid (2,4-D). While addition of cytokinin inhibited embryogenesis. Role of amino acids were also evaluated, among the four amino acids viz: Proline, Glutamine, Asparagine and Serine, Proline and Glutamine at lower concentration favored embryogenesis. Morphogenesis of somatic embryos was observed about a week after transfer on to the MS medium containing 6-benzylaminopurine (BAP) and a-napthalene acetic acid (NAA). Elongated shoots were transferred on to the medium containing indole-3-butyric acid (IBA) for rooting. Complete plantlets thus obtained were successfully hardened and grown to maturity.
KEYWORDS: Somatic embryos: plantlet regeneration: Carthamus tinctorius: cotyledons, hypocotyl
Download this article as:| Copy the following to cite this article: Talat, K. and Anwar, S. Y.High Frequency Somatic Embryogenesis and Plantlet Regeneration Via Somatic Embryos in Safflower (Carthamus Tinctorius L.). Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Talat, K. and Anwar, S. Y.High Frequency Somatic Embryogenesis and Plantlet Regeneration Via Somatic Embryos in Safflower (Carthamus Tinctorius L.). Biosci Biotech Res Asia 2010;7(1).. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9273 |
Introduction
Present era of biotechnology based agricultural science has emerged in response to the limitations of conventional plant breeding techniques. Somatic embryos in view of the single cell origin may be used as “Target Tissue” in genetic transformation studies. However, a reproducible method of induction of somatic embryos and an efficient and reproducible regeneration protocol from the Somatic embryos is a prerequisite in utilizing the power and potential of this technology. Although, excellent progress has been made in obtaining transgenic plants from most of the oil seed crop plants, no significant progress has been made in this direction in safflower, most likely due to non availability of an efficient regeneration protocol from the target tissue i.e somatic embryos. According to Nikam & Shitole (1999), regeneration frequency and rooting capacity of safflower is very low. However, protocols of plantlet regeneration from cotyledonary explants for Indian (George & Rao, 1982; Tejovathi & Anwar, 1987, 1993) Turkish (Dilek Ba alma et al, 2008) and American safflower cultivars (Charleen & William, 1996) have been developed. Besides, protocol for in vitro induction of capitulum (Tejovathi & Anwar, 1984; Yashodra et al, 1993) and in vitro induction of androgenic haploids (Prasad et al, 1990, 1991) for Indian safflower cultivars have also been developed. In addition, somaclonal variation (Seeta et al, 2000) and in vitro pollen (Seeta et al, 1999) as novel sources of genetic variability in safflower have also been reported.
Present study describes in detail induction of somatic embryos and plantlet regeneration from somatic embryos.
Materials and Method
Plant material
Seeds of ten different Genotypes viz. Manjira, A-1, HUS-305, Tara, GMU-826, GMU-827, APRR-3, Bhima, Co -1, KAS-1 of safflower (Carthamus tinctorius L) were procured from the Directorate of oil seed Research (I.C.A.R.), Rajendranagar, Hyderabad, grown and maintained in the Plant Genetics experimental farm, Department of Genetics, Osmania University were used in the present study.
Surface sterilization and inoculation
Seeds of ten different genotypes were surface sterilized with 0.1% Hgcl2 in sterile flasks under aseptic conditions for 8-10 minutes and were washed thoroughly with sterile distilled water for three times each of 5 minutes duration. Seeds were then germinated on sterilized wet filter paper bridges. Cotyledon, hypocotyls and root of seven days old seedlings were used as explants for the induction of somatic embryogenesis. The explants were cut into small pieces and about 4-5 explants/tube were inoculated onto MS and B5 medium supplemented with different phytohormones viz: 2,4-D, 2,4,5-T, NAA and IAA at different concentrations (0.5 mg-1 to 2.0 mg-1) for the induction of somatic embryogenesis. Cytokinins such as 6-Benzylaminopurine (BAP) at four different concentrations (0.5, 1.0, 1.5 and 2.0 mg-l) and kinetin (KN) at two different concentrations 0.1 mg-l and 0.5 mg-l were incorporated in the MS medium. Amino acids viz: proline, Glutamine, Serine and Asparagine at two different concentrations 0.1 mg-l and 0.5 mg-l were also incorporated into the medium to observe the embryogenic response. All the experiments were carried out in a sterile chamber and the culture tubes were incubated under cool, white and continuous light at 25 ± 1°C with 50-60% relative humidity. Fluorescent tubes of 2000-2500 lux provided the light. All the cultures were maintained in a minimum of three replicates.
To observe the morphogenic response, fully developed globular to torpedo shaped somatic embryos were transferred into medium, supplemented with different concentrations of auxins and cytokinins. Shoot elongation was achieved by regular sub culturing of the shoot buds into the same medium and for rhizogenesis elongated shoots were transferred to MS half strength medium supplemented with different concentrations of indole-3-butyric acid (IBA) for rooting.
Regenerated plants obtained from the somatic embryo of Manjira, A-1 and HUS-305 with well-developed roots were taken out of the medium, washed with water to remove agar medium and transferred to pots containing vermiculite. Potted plants were kept at a temperature of 25-26°C. the plants thus established were grown to maturity.
Data Analysis
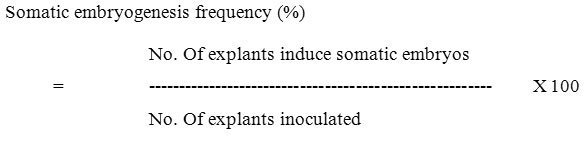
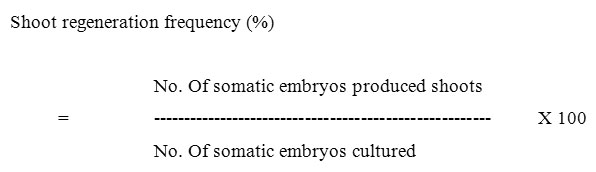
The experimental results were calculated according to the following formulae.
)

Statistical Analysis
Statistical analysis is done according to the Snedecor and Cochran (1980). Analysis of Variance (two ways, three ways, and four ways) is calculated as per the requirement.
F- test of significance:
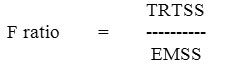
If the calculated f value is >= f-tabulated value, it is inferred that significant difference exists. Comparison of means between treatments is done with the help of critical difference.
CD =’t’ table value at Err.df X Ö 2x EMSS/n.
Results and discussion
Successful induction of somatic embryos either directly or indirectly and subsequent plantlet regeneration depends upon the composition of the nutrient media, choice of the explants besides genotype (Buckley et al, 2000). Among the two different media, B5 medium showed relatively low frequency of embryogenesis while MS medium showed maximum embryogenic response. Superiority of MS medium over B5 used in the study may be attributed to the higher nitrate content in the MS medium. The source and amount of total nitrogen in the basal medium in particular are the critical factors for in vitro response of the explant (Dilek Ba alma et al, 2008). The highest rate of embryogenesis was observed in groundnut in the modified MS medium (Sabitha & Reddy, 1996). Somatic embryogenesis in groundnut was also induced directly without any intervening callus phase on MS medium (Venkatchalam et al, 1997). Somatic embryogenesis in Niger was induced on LS medium (Venkatesham & Reddy, 1996). However, the degree of response varied with the genotype and the explants.
Among the ten genotypes tested HUS-305 exhibited the maximum response followed by Tara, Manjira and A-1 where as, other genotypes showed only callus induction without any differentiation into somatic embryos. Observations made in this study clearly suggest that the genotypes used critically influenced the in vitro response towards somatic embryogenesis. Somatic embryogenesis in Soybean is strongly genotype dependent and has been linked to the genetic background of the cultivars. Of the 20 lines of northern adopted cultivars of soybean tested for the induction of somatic embryos, it was noticed that although most cultivars have the ability to regenerate in vitro, many cultivars have a very poor response (Buckley et al, 2000). Genotypic differences in the induction of somatic embryos have also been reported in groundnut (Sabitha & Reddy 1996).
Of the three different explants viz: Cotyledon, Hypocotyl and Root used, It is obvious from the data recorded (Table-1 & 1a) that the percent induction of somatic embryos was maximum (84.85%) with the hypocotyl explant. whereas with cotyledonary explants, % frequency of induction of somatic embryos was 23.48%, histological studies of these embryos revealed all the stages globular, heart and torpedo shaped structures. (Fig-1D) while, the root explants completely failed to respond. The analysis of variance (ANOVA) revealed that the variation associated with the explants, genotypes and their interaction is highly significant. Successful induction of somatic embryos depends on the explants as well as on the nutritional and hormonal components in the culture media (Sabitha & Reddy 1996). In Niger, immature leaves were the most responsive towards the induction of somatic embryos (Venkatesham & Reddy, 1996) while cotyledonary explant yielded maximum frequency of somatic embryos in soybean (Buckley et al, 2000). In sunflower, immature embryo was found to be efficient in the induction of somatic embryos (Finer, 1987).
Table 1 Role of genotypes and explants for the induction of somatic embryogenesis in Safflower.
| Cotyledons | Hypocotyl | Root | |
| Genotypes | % responded | % responded | % responded |
| Manjira | Callus | 64.29 | NR |
| A-1 | 17.86 | 50 | NR |
| HUS-305 | 22.22 | 84.85 | NR |
| Tara | 23.49 | 67.59 | NR |
Table 1a. Analysis of variance of the role of genotype and explant for induction of somatic embryogenesis in safflower.
| Source of | d.f. Mean of CD CD | ||||
| Variation |
|
squares
F.rati o S.E.di f.
(0.05%) (0.01%) |
|||
| Genotypes | 3 | 458.83 | 38.37** | 1.99
5.83 |
4.23
|
| Explants | 1 | 17153.74 | 1434.39** | 1.41
4.12 |
2.99
|
| Interaction | 3 | 544.94 | 45.57** | 2.82
8.25 |
5.98
|
| Error | 16 11.96 | ||||
Table 2: Role of explants & auxins for the induction of somatic embryogenesis in different genotypes of Safflower.
| Genotypes | Explants | Auxins |
0.5 mg/l
Mean response (%) |
1.0 mg/l
Mean response (%) |
1.5 mg/l
Mean response (%) |
2.0 mg/l
Mean response (%) |
|
Manjira |
Cotyledon | 2,4-D | 18.67 | 35.90 | 28.65 | 20.55 |
| 2,4,5-T | 7.79 | 10.42 | 14.59 | 6.78 | ||
| Hypocotyl | 2,4-D | 38.89 | 28.76 | 27.54 | 10.16 | |
| 2,4,5-T | 22.22 | 26.19 | 64.29 | 41.67 | ||
| A-1 | Cotyledon | 2,4-D | 6.65 | 10.21 | 12.22 | 12.16 |
| 2,4,5-T | 6.67 | 11.89 | 17.86 | 10.00 | ||
| Hypocotyl | 2,4-D | 44.44 | 54.17 | 39.45 | 28.22 | |
| 2,4,5-T | 29.52 | 48.04 | 50.00 | 40.00 | ||
| HUS-305 | Cotyledon | 2,4-D | 25.00 | 22.22 | 15.08 | 12.82 |
| 2,4,5-T | 24.44 | 31.88 | 22.22 | 16.00 | ||
| Hypocotyl | 2,4-D | 43.06 | 30.08 | 34.75 | 30.86 | |
| 2,4,5-T | 32.22 | 76.67 | 84.85 | 64.71 | ||
| Tara | Cotyledon | 2,4-D | 22.92 | 10.14 | 30.95 | 15.74 |
| 2,4,5-T | 21.05 | 21.05 | 23.48 | 20.67 | ||
| Hypocotyl | 2,4-D | 17.95 | 18.52 | 17.42 | 12.04 | |
| 2,4,5-T | 13.16 | 30.30 | 67.59 | 37.12 |
NAA & IAA showed least embryogenic response, hence not included in the data.
Table 3: Effect of Aminoacids for the induction of somatic embryogenesis in different genotypes of Safflower.
|
Genotypes |
Explants |
Amino acids |
||
| concentration | ||||
|
Manjira |
Cotyledon | Proline | 38.27 | 26.67 |
| Glutamine | 17.17 | 10.08 | ||
| Asparagin | 3.47 | 0.00 | ||
| Serine | 0.00 | 0.00 | ||
| Hypocotyl | Proline | 37.78 | 32.18 | |
| Glutamine | 27.33 | 23.48 | ||
| Asparagin | 12.90 | 9.55 | ||
| Serine | 0.00 | 0.00 | ||
| A-1 | Cotyledon | Proline | 42.59 | 29.17 |
| Glutamine | 30.63 | 23.53 | ||
| Asparagin | 11.90 | 8.18 | ||
| Serine | 8.00 | 7.35 | ||
| Hypocotyl | Proline | 17.65 | 15.63 | |
| Glutamine | 13.27 | 6.13 | ||
| Asparagin | 0.00 | 0.00 | ||
| Serine | 0.00 | 0.00 | ||
| HUS-305 | Cotyledon | Proline | 49.55 | 28.00 |
| Glutamine | 48.65 | 41.23 | ||
| Asparagin | 12.00 | 13.65 | ||
| Serine | 6.67 | 4.20 | ||
| Hypocotyl | Proline | 14.29 | 13.10 | |
| Glutamine | 0.00 | 0.00 | ||
| Asparagin | 6.53 | 0.00 | ||
| Serine | 3.74 | 1.72 | ||
| Tara | Cotyledon | Proline | 27.08 | 21.09 |
| Glutamine | 14.00 | 8.00 | ||
| Asparagin | 0.00 | 0.00 | ||
| Serine | 5.93 | 6.00 | ||
| Hypocotyl | Proline | 14.81 | 9.86 | |
| Glutamine | 0.00 | 0.00 | ||
| Asparagin | 8.25 | 9.90 | ||
| Serine | 0.00 | 0.00 | ||
Table 4: Morphogenic response of somatic embryos in MS medium with various concentrations of Auxins and Cytokinin.
| Hormone conc. mg/l | No. of S.E. cultured | No. of S.E. responded |
Type of response |
regeneration
frequency (%) |
|
BAP |
|
|
|
|
|
0.5 |
80 |
45 |
Slight greening |
56.25 |
|
1.0 |
75 |
40 |
Slight greening |
53.33 |
|
1.5 |
60 |
30 |
Greening of embryos |
50.00 |
|
2.0 |
70 |
32 |
Small shoot buds |
45.71 |
| BAP+NAA |
|
|
|
|
|
0.5 + 0.1 |
85 |
72 |
Shoot bud and leaf like structure |
84.71 |
| 1.0 + 0.1 | 80 | 65 | Shoot bud | 81.25 |
|
0.5 + 0.5 |
70 |
45 |
Shoot buds and small roots | 64.29 |
|
1.0 + 0.5 |
65 |
38 |
Shoot buds and small roots | 58.46 |
| BAP+IAA |
|
|
|
|
|
0.5 + 0.1 |
6o |
08 |
Slight greening | 13.33 |
|
1.0 + 0.1 |
50 |
06 |
Slight greening | 12.00 |
|
0.5 + 0.5 |
30 |
Non embryogenic callus |
Shoot buds and small roots |
— |
|
1.0 + 0.5 |
42 |
Non embryogenic callus |
Shoot buds and small roots |
— |
Table 5: Rhizogenic response of regenerated shoots in different genotypes of safflower.
|
Genotype |
Hormone conc.(mg/l) |
No. of shoots placed for rooting |
No. of shoots with roots |
Regeneration frequency (%) |
|
Manjira |
IBA |
|
|
|
|
|
1.0 |
10 |
NR |
– |
|
|
2.0 |
15 |
02 |
13.33 |
|
|
3.0 |
10 |
02 |
20.00 |
|
|
4.0 |
12 |
04 |
33.33 |
|
|
5.0 |
21 |
08 |
38.09 |
|
A-1 |
IBA |
|
|
|
|
|
1.0 |
12 |
NR |
– |
|
|
2.0 |
12 |
02 |
16.66 |
|
|
3.0 |
15 |
03 |
20.00 |
|
|
4.0 |
10 |
03 |
30.00 |
|
|
5.0 |
20 |
07 |
34.62 |
|
HUS-305 |
IBA |
|
|
|
|
|
1.0 |
8 |
NR |
—- |
______________
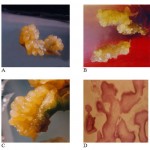 |
Figure 1: A.B.C.D
|
The role of four different auxins viz: 2,4-D, 2,4,5-T, NAA and IAA in different concentrations ranging from 0.5 mg-1 to 2.0 mg-1 on the induction of somatic embryogenesis was also evaluated. Among the four auxins tested, 2,4,5-T showed the highest embryogenic efficiency followed by 2,4-D. Hypocotyl explants of the genotype HUS-305 with 1.5 mg-1 2,4,5-T showed the maximum (84.85%) embryogenic response (Fig-1A) followed by Tara, (Fig-1B) Manjira and A-1. Increasing or decreasing concentrations of 2,4,5-T decreased the embryogenic response (Table-2 & 2a). ANNOVA revealed that variation associated with the effects of auxin, its concentration and their interaction is highly significant at 1% level. These observations are in agreement with the findings in chickpea (Sagare et al, 1993). Other auxins (NAA and IAA at all concentrations) used in this study failed to induce somatic embryos. However, their higher concentrations (1.5 mg-1 and 2.0 mg-1) resulted in the formation of embryogenic calli and/or rooting.
 |
Figure 2: A.B.C.D
|
Effect of combination of auxins (2, 4-D and 2, 4, 5-T) and cytokinin viz. kinetin on the induction of somatic embryogenesis was also studied. Different concentrations of 2, 4-D (0.5 mg-1 to 2.0 mg-1) and 2, 4, 5-T (0.5 mg-1 to 2.0 mg-1) in combination with kinetin (0.1 mg-1 and 0.5 mg-1) increased the callusing frequency with very low frequency of induction of somatic embryos. Results obtained clearly indicated that the presence of cytokinin inhibited the process of embryogenesis. Addition of cytokinin viz. kinetin with two different concentrations (0.1 mg-1 and 0.5 mg-1) was found to inhibit somatic embryogenesis in groundnut (Reddy & Reddy, 1993). For the induction of somatic embryos in sesamum 2,4-D alone was reported to be the most effective (Jaya & Balan, 1997) . In alfalfa, the inductive treatment is most commonly 2,4-D but other auxin such as 2,4,5-T was also effective (Lai & Mc Kersie, 1994). However, the highest rate of embryogenesis was observed in groundnut in the modified MS medium containing 2,4-D and KN (Sabitha & Reddy, 1996).
Organic components such as amino acids particularly proline present in the medium can also modulate the process of embryogenesis (Shetty & Mc Kersie, 1993). Keeping this in view, in the present study, effect of four different amino acids viz. Proline, Glutamine, Asparagine and Serine, at two different concentrations (0.5 mgl-1 and 1.0 mgl-1) was evaluated to know their influence on embryogenic response using both cotyledon and hypocotyl explants. It is obvious from the data shown (table-3), that both proline and glutamine at lower concentration increased the frequency of somatic embryos while Asparagine and Serine did not show much positive response. However among proline and glutamine, proline is more effective than glutamine. Another aspect of this study is that the positive response of both proline and glutamine in the induction of somatic embryogenesis is confined only to cotyledonary explants (Fig-1C). Addition of 0.5 and 1.0 mM of the amino acid in the medium increased the proliferation of embryogenic callus in Maize (Suprasanna et al, 1994). However, no marked differences were observed between the basic medium and the medium containing 0.1mM amino acids in triticum aestivum x Leymus angustus (Zohreh et al, 1993). Proline has been shown to improve somatic embryogenesis in maize (Armstrong & Green 1985). Argenine and glutamic acid did not increase the formation of embryogenic callus in sugarcane (Ho & Vasil, 1983). In alfalfa, glutamine plays regulatory and nutritive role in somatic embryo maturation (Lai et al, 1992).
Since direct germination of somatic embryos into a plant was found to be poor, plantlet regeneration from somatic embryos was achieved in a two step process: Shoot bud initiation from the somatic embryos is the first step followed by rhizogenesis of the well-developed shoots. Fully matured somatic embryos were isolated and were transferred to regeneration medium with different plant growth regulators viz. BAP (0.5 mg-1 to 2.0 mg-1) NAA and IAA (0.1mg-1 and 0.5 mg-1). Initiation of shoot bud (Fig.2A) was observed on MS medium supplemented with 0.5 mg-1 BAP and 0.1mg-1 NAA. The highest morphogenesis was observed in the genotype Manjira (84.71%) followed by A-1 (71.43%) HUS-305 (55%) and Tara (41.67%). Increasing concentrations of BAP 1.0 mg-1 to 2.0 mg-1 and NAA (0.1mg-1 to 0.5 mg-1) decreased the shoot regeneration frequency. When BAP (0.5 mg-1) was supplemented with IAA, there was very poor shoot regeneration response in all the genotype tested (table-4). Shoot elongation was obtained by regular sub culturing the shoot buds on the same medium (Fig-2B). In Arachis hypogea mature somatic embryos of the cultivar ICG 8123 were regenerated with a frequency of 4.2% on MS basal medium where as the other genotypes ICG 799 and ICG 1908 turned brown and failed to develop into complete plant-lets (Sabitha & Reddy, 1996).
In an another study in A. hypogea, germination of somatic embryos was observed on half-strength MS basal medium without growth regulators after two weeks of cultures (Reddy & Reddy, 1993). Somatic embryo maturation and germination was achieved on MS basal as well as MS supplemented with 1.0 mg-1 Kinetin in Banium persicum (Wakhlu et al, 1990).
For rooting of the regenerated shoots of safflower, in the present study IBA was used with five different concentrations (1.0 mg-1 to 5 mg-1) to see its effect on rooting response. The shoots of Manjira developed 6 to 8 elongated roots on the medium containing 5.0 mg-1 IBA (Fig-2C). The frequency of rooting was highest in the Genotype Manjira (38.09), followed by A-1 (34.62) and HUS-305 (26.67), while rooting response was completely absent in the genotype Tara. Complete plantlets with well developed roots were removed from the tubes/bottles and were washed in the running tap water then these plantlets were transferred to plastic cups containing sterile peet math soil and later established into pots (Fig-2D) and were maintained in the green house.
Conclusion
Standardization of the protocol reported in the present study on induction of somatic embryogenesis and plantlet regeneration from somatic embryos will go a long way in the utilization of somatic embryos as target tissue keeping in view their single cell origin, in genetic transformation studies and in regeneration of transgenics (data under publication).
References
- Alma, D.B.; Serkan, U.; Semra, M.; Ozer, K. TDZ x IBA induced shoot regeneration from cotyledonary leaves and invitro multiplication in safflower. African J Biotech 7: 960-966 (2008).
- Armstrong, C. L.; Green, C. E. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 164:207-214 (1985).
- Buckley, D. J.; Dangi, O. P. Finstad, K. I.; Jing, S. Q.; Peng, X. B.; Tian, L. N. Invitro regeneration and gene transfer in legumes. ECORC- Advances in research, Invitro regeneration and synthetic seed technology (2000).
- Charleen, M. Baker.; William, E. Dyer. Improvements in rooting regenerated safflower shoots. Pl Cell Rep 16: 106-110 (1996).
- Finer, J. J. Direct somatic embryogenesis and plant regeneration from immature embryos of hybrid sunflower on a high sucrose containing medium. Pl breed 107: 280-287 (1987).
- Gamborg, O. L.; Miller, R. A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 50: 151-158 (1968).
- George, L. Rao, P. S. In vitro multiplication of safflower. Proc Ind Natl Sci Acad, 48: 791-794; 1982
- Ho, W.; Vasil, I. K. Somatic embryogenesis in sugarcane the morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasma 118: 169-180 (1983).
- Jaya Mary, R.; Jayabalan, N. Influence of growth regulators on somatic embryogenesis in sesame. Pl Cell Tiss Org Cult 49: 67-70 (1997).
- Lai, F. M.; Mc Kersie, B. D. Regulation of storage protein synthesis by nitrogen and sulphur nutrients in alfalfa somatic embryos. Plant Sci 103: 209-221 (1994).
- Lai, F. M.; Senaratna, T.; Mc Kersie, B. D. Glutamine enhances storage protein synthesis in Medicago sativa L. somatic embryos. Plant Sci 87: 69-77 (1992).
- Murashige, T.; Skoog, T. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-497 (1962).
- Nikam, T.D.; Shitole, M.G. Invitro culture of safflower L cv. Bhima initiation, growth optimization and organogenesis. Pl cell tiss org cult 5: 15-20 (1999).
- 14 Prasad, B. R.; Khadeer, M. A.; Seeta, P.; Anwar, S. Y. Influence of genotype and cold pretreatment on anther culture response in safflower. Ind J Expt Biol 28: 924-927 (1990)
- 15 Prasad, B. R.; Khadeer, M. A.; Seeta, P.; Anwar, S. Y. In vitro induction of androgenic haploids in safflower. Pl Cell Rep 10: 48-51 (1991).
- 16 Reddy, R. L.; Reddy, G. M. Factors affecting direct somatic embryogenesis and plant regeneration in groundnut. Ind J Exp Biol 31: 57-60 (1993).
- 17 Sabitha Rani, A.; Reddy, G. M. Induction of somatic embryogenesis from young leaflets of cultivated and wild species of groundnut. Ind J Expt Biol 34: 569-571 (1996).
- 18 Sagare, A. P.; Suhasini, K.; Krishnamurthy, K. V. Plant regeneration via somatic embryogenesis in chick pea. Pl Cell Rep 12: 652-655; 1993.
- 19 Seeta, P.; Talat, K.; Anwar, S. Y. Somaclonal variation – An alternate source of genetic variability in safflower, J Cytol Genet 1: 127-135; 2000.
- 20 Seeta, P.; Talat, K.; Anwar, S. Y. In vitro pollen(s)- novel source of genetic variability in safflower. Ind J Expt Biol 37: 491-495; 1999.
- 21 Shetty, K.; Mc Kersie, B. D. Proline, thioproline and potassium mediated stimulation of somatic embryogenesis in alfalfa. Pl Sci 88: 185-193; 1993.
- Snedecor, G.W.; Cochran, W.G. Statistical methods, the Iowa State University press, IOWA, USA. 1967
- Suprasanna, P.; Rao, K. V.; Reddy, G. M. Embryogenic callus in maize: genotypic and amino acid effects. Cereal Research Communications 2: 79-82; 1994.
- Tejovathi, G.; Anwar, S. Y. Plantlet regeneration from cotyledonary explants of safflower In: Reddy G M (ed) Plant cell and Tissue culture of economically important plants. 347-355; 1987.
- Tejovathi, G.; Anwar, S. Y. In vitro induction of capitula from cotyledonary explants of safflower. Pl Sci Lett 36: 165-168; 1984.
- Tejovathi, G.; Anwar, S. Y. Trichlorophenoxy propionic acid induced rhizogenesis in safflower. Proc Ind Natl Sci Acad B 59: 633-636; 1993.
- Venkatachalam, P.; Kavi Kishor, P. B.; Jaya balan, N. High frequency somatic embryogenesis and efficient plant regeneration from hypocotyl explants of groundnut. Current Science, 72: 271 – 275; 1997.
- Venkatesham, G.; Reddy, T. P. Plant regeneration from immature zygotic embryo cultures of niger. Ad Pl Sci 9: 15-20; 1996.
- Wakhlu, A. K.; Sangita N.; Barna, S. K. Somatic embryogenesis and plant regeneration from callus cultures of Banium persicum Boiss. Pl Cell Rep 9: 137-138; 1990.
- Yashodra, Y.; Tejovathi, G.; Anwar, S. Y. Effect of Gibberellic and Absicisic acids on in vitro flowering in safflower. J Cytol Genet 28: 137-140; 1993.
- 31. Zohreh, T.; Arian P.; Andre C. Somatic embryogenesis and plant regeneration in Triticum aestivum x Leymus angustus F1 hybrids and the parental lines. Pl Cell Rep 9: 204-206; 1993.

This work is licensed under a Creative Commons Attribution 4.0 International License.





