How to Cite | Publication History | PlumX Article Matrix
Immunological Diagnosis of Human Immunodeficiency Virus: Experience Based Report
Amit K. Dutta and Sanju Varghese
Department of Biotechnology, Rungta College of Science and Technology, Durg India.
Corresponding Author E-mail:adjnchrc@yahoo.com
ABSTRACT: The main purpose of this work is to detect the HIV response rates at Out Patients Department (OPD) of Apollo Hospital, Bilaspur, particularly those who are coming from the rural area of Chhattisgarh State. Blood plasma samples of 120 patients were taken and Tri-Dot and ELISA test for the detection of HIV. Out of 120 OPD patients of Apollo hospital, 20 were HIV positive i.e., 16.6%. It was also seen that maternal transmission of HIV accounts for more than 90% of all HIV infection in infants & children. It was concluded from demographical study that life expectancy of HIV positive pregnant woman depends upon the antenatal clinical treatment. The mean time from seroconversion to onset of disease is approximately 9 years. Perinatally infected infants’ progress faster. Signs of AIDS can be seen by 5 months in more than 80% of seropositive children. Since the work is small but definitely it will help in future to study the statistical population genetics of Chhattisgarh state.
KEYWORDS:
Blood Sample; Tri-Dot; ELISA; Pre-Test & Post-Test Counseling
Download this article as:| Copy the following to cite this article: Dutta A. K, Varghese S, Immunological Diagnosis of Human Immunodeficiency Virus: Experience Based Report. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Dutta A. K, Varghese S, Immunological Diagnosis of Human Immunodeficiency Virus: Experience Based Report. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9757 |
Introduction
Disease and death have always held the attention of the human mind. Ancient human ascribed them to divine wrath and other supernatural forces. Later, other concepts such as the effect of the environment, of bodily constitution and of faulty diet were proposed1. Human Immunodeficiency Virus (HIV) is a lentivirus that can lead to Acquired Immunodeficiency Syndrome (AIDS), a condition in human in which the immune system begins to fail, leading to life threatening opportunistic infection. Previous names for the virus include human T- lymphotropic virus-III (HTLV-III), lymphadenopathy-associated virus (LAV) and AIDS- associated retrovirus (ARV)5. AIDS stands for Acquired Immune Deficiency Syndrome, a retroviral disease characterized by profound immunosuppression that leads to an opportunistic infection secondary neoplasm & neurological manifestations. It is caused by a virus called HIV, the Human Immunodeficiency Virus. The climax came in 1981 when AIDS was identified in the USA and began its pandemic spread. Unceasing vigilance appears essential to protect humans from microbes2.
HIV is a complex retrovirus around 120nm in diameter and roughly spherical and belong to the family Retroviridae. It uses the enzyme reverse transcriptase to transform RNA to DNA.HIV can be classified as two types HIV-1 & HIV-2. It has several major genes coding for structural proteins that are found in all retroviruses, and several nonstructural genes that are unique to HIV. It consists of outer envelope, core shell and inner core. Other associated proteins are gp41, GP120 in outer envelope, P17 in outer layer of core shell and P24 in inner layer of core shell. Inner core contain P10 & P32. Structural gene has 3 major groups: Gag, Pol gene and Env gene. Gene products Gag gene are: P17, P24, P9 & P7; products of Pol gene are P64 (having reverse transcriptase & RNase activity), P51, P10, P32; Env gene codes for gp41 (Trans membrane protein) & gp120 (envelop protein & CD4). Tat code for Trans- Activator of Transcription, Rev Code for Regulator of Virion & Nef stands for Negative factor, Vif for Viral infectivity factor, Vpu for Viral protein U, Vpr for Viral protein R.
Although AIDS was first described in the United States, AIDS has now been reported from more than 193 countries around the world & the pool of HIV infected persons in Africa & Asia is large & expanding. Epidemiological studies in the united sates have been identified in five groups of adults at risk for the developing AIDS4.
The clinical care of people infected with human immunodeficiency virus (HIV) has been substantially affected by the introduction of HIV-specific protease inhibitors (Pls). The 4 Pls available are saquinavir mesylate, ritonavir, indinavir sulfate and nelfinavir mesylate. The Pls have emerged as critical drugs for people with HIV infection. Optimal use involves combination with reverse transcriptase inhibitors. Resistance develops to each agent, and cross- resistance is likely. These agents must be used at full doses with attention to ensuring patient compliance. The expense of these agents may be offset by forestalling disease progression and death and returning people to productive life. Selecting the initial PI must be individualized and factors to consider include proven activity, possible toxicities, dosing regimens, drug interactions and costs3.
Materials and Methods
Blood plasma samples of 120 patients were taken and Tri-Dot and ELISA test for the detection of HIV.
Tri Dot Test
Instrument and Chemicals
Hand gloves, Poly bags, Tri- Dot Test device, Buffer solution and Conjugate.
After setting all the necessary condition and facilities of the working lab added 3 drops of buffer solution into the Tri Dot Test Device. To it were added 1 drop of serum or plasma and 5 droops of Buffer solution. After few minutes added 2 drops of conjugate solution and 5 drops of Buffer solution. If only dot appear on control then specimen sample is non reactive but if dot appear on control and on HIV-1 or HIV-2 then the specimen is reactive for antibodies of HIV-1 and HIV-2 respectively.
ELISA Test
Instrument & Chemicals
Hand Gloves, Poly Bags, micropipettes, Microtips, ELISA Reader, Glass ware, Timer, ELISA, Washer, Vortex, mixer, Paper Towels. Micro Lisa- HIV Strip Plates, Sample Diluents, enzyme conjugate Concentrate, Conjugate Diluents, Wash Buffer, TMB Concentrate substrate, control and stop solution.
ELISA was performed as per the standard protocol and the reactivity was analyzed using ELISA Reader.
HIV testing carried out on a voluntary basis with appropriate pre-test & post-test counseling is considered to be better strategy and is in line with the WHO guidelines on HIV testing. The emergence and pandemic spread of the acquired immunodeficiency syndrome (AIDS) have posed the greatest challenge to public health in modern times. The main purpose of my research is to detect the HIV response rates at Out Patients Department(OPD) of Apollo Hospital, Bilaspur, particularly those who are coming from the rural area of Chhattisgarh State.
Observation and Results
Table 1: Analyses of HIV positive patients on the basis of age Group.
| Age group | No. of Patients | Positive | Negative |
| Below10 | 1 | 1 | 0 |
| Below20 | 3 | 3 | 0 |
| Below30 | 28 | 3 | 25 |
| Below40 | 41 | 6 | 35 |
| Below50 | 36 | 6 | 30 |
| Below60 | 11 | 1 | 10 |
| Total | 120 | 20 | 100 |
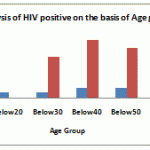 |
Figure 1
|
This work has been carried out on immunological diagnosis of HIV at Apollo Hospital; Bilaspur (C.G.) Total 120 patients were selected for this work. The main purpose of study was to determine and diagnose for HIV through two important techniques. Blood plasma was isolated for this study. Out of 120 OPD patients of Apollo hospital, 20(16.6%) were HIV positive. It was also seen that maternal transmission of HIV accounts for more than 90% of all HIV infection in infants and children. Life expectancy of HIV positive pregnant woman depends upon the antenatal clinical treatment. Despite possible co-factors associated with lifestyle, HIV infected person’s progress to AIDS at a remarkably similar rate. The mean time from seroconversion to onset of disease is approximately 9 years. Perinatally infected infants progress faster. Signs of AIDS can be seen by 5 months in more than 80% of seropositive children.
Discussion
HIV is a lentivirus (a member of the retrovirus family) that can lead to acquired immunodeficiency syndrome (AIDS), a condition in humans in which the immune system begins to fail, leading to life-threatening opportunistic infections. HIV is the causative agent of AIDS was identified in 1983, following the first report case of AIDS in 1981, 1982. HIV belongs to the class Retrovirus that store genetic information as two strands of RNA and carry a special enzyme Reverse Transcriptase. HIV has surface knobs made of glycoprotein 120 complementary to the receptors CD4 on host T cell. Both fit together like lock and key. Viral DNA after reverse transcription integrates with host T cell nucleus and virus starts replicating and changing. The body defenses the course of HIV infection when virus is not replicating. The body defense reacts to the presence of HIV by mounting an effective & is the stage of seroconversion. Period earlier to seroconversion is called window period. During this period virus is actively replicating, individual is infected & infectious but HIV specific antibodies cannot be detected in the serum. As the HIV disease progresses more and more T cells get infected. The activated T cells in lymph node also get infected; as a result the FDC network breaks down trying to combat such high levels of the virus. This process continues till FDC network completely breaks down and other viruses, bacteria & fungi are able to enter and cause infections. Gradually the levels of HIV become so high that it infects and destroys T lymphocytes at a faster rate than the body is able to replenish them. Eventually AIDS sets in indicating the advanced stage of HIV infection.
The AIDS or HIV infection is caused by HIV virus family of retro virus & sub group of lentivirus. The diagnosis of HIV infection depends upon the demonstration of antibodies of HIV & or the direct detection of HIV or one of its component. Various tests are available in the diagnosis of HIV infection or AIDS. Screening test and confirmatory test are the two important methods of testing. Tri dot is a rapid screening test for HIV infection and the standard screening test for HIV infection is the ELISA. The most accurate diagnosis of HIV infection in early infancy comes from test that shows the presence of virus itself in the body. From the following test it was concluded that the test performed by using Tri Dot & ELISA are the method through which we can diagnosis HIV patient from the above two HIV Tri Dot test is rapid test & sensitive too.
Conclusion
The main purpose of this work was to detect the HIV response rates at out patients department (OPD) of Apollo Hospital, Bilaspur, particularly those who are coming from the rural area of Chhattisgarh State. It was already proved by the well known scientists that the emergence and pandemic spread of the AIDS have posed the greatest challenge to public health in modern times. From the entire work, it can be concluded that out of 120 OPD patients of Apollo Hospital 20 were HIV positive i.e., 16.6%.
HIV positive patients in Apollo Hospital, Bilaspur were more in the age group of 30-50. The mean time from seroconversion to onset of disease is approximately 9 years. Perinatally infected infants’ progress faster. Signs of AIDS can be seen by 5 months in more than 80% of seropositive children.
Since the work is a small one and a beginning of our achievement, it was not possible to give a final clear cut conclusion and statement. But definitely it will help in future to study the statistical population genetics of the rural area of Chhattisgarh state.
Acknowledgement
We are grateful to Mr. T. Mohan Raja, Dr. Manju Barua, Apollo Hospital, Bilaspur, C.G. for allowing to carry out the work there. We owe our deep gratitude towards Prof (Dr.) G. D. Sao, Prof. J. P. Sharma & Mr. Sanjeev Shukla for providing necessary facilities in RCST, Durg. We are also thankful to all the person of rural area of CG state without whom it would not possible to complete this work.
References
- Ananthanarayan R, Paniker CKJ. Text Book of Microbiology. Orient Longman Private Limited. 6(1): 1- 3(2000).
- Ananthanarayan R, Paniker CKJ. Text Book of Microbiology. Orient Longman Private Limited. 6(62): 539-554(2000b).
- Deeks SG, Smith M, Holodniy M and Kahn J.O. HIV-1 Protease Inhibitors. A Review for Clinicians. The Journal of the American Medical Association. 277(2): 1(1997).
- Kallings LO. ‘The First Postmodern Pandemic : 25 Years of HIV/AIDS’. J Intern Med. 263(3): 218-243(2008).
- Rick Sowadsky.“What is HTLV-III?. (199-02-24). Retrieved (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.





