Manuscript accepted on : February 02, 2010
Published online on: 28-05-2016
Non- Enzymic Antioxidant Activity of Clitoria Ternatea Leaf Extracts in Vitro
A . Jayachitra1 and P. R. Padma2
1Department of Biochemistry and Bio-technology, Sourashtra College, Madurai - 625 004 India.
2Reader,Department of Biochemistry, Biotechnology and Bioinformatics, Avinashilingam University, Coimbatore India.
Correspondng Author E-mail: jchitra21@gmail.com
ABSTRACT: Non-enzymic antioxidants were analyzed in both blue flowered leaf and white flowered leaf of Clitorea ternatea. The in vitro model used in the study as alternatives to live animals, was goat liver slices. The liver slices exposed to different treatments were incubated for one hour at 37°C with very mild shaking. The non- enzymic activity was analyzed by using goat liver slices, both blue flowered leaf and white flowered leaf of Clitorea ternatea and H2O2 as oxidant. The results showed that the white flowered leaves had higher content of all the non-enzymic antioxidants analyzed than the blue flowered ones.
KEYWORDS: Clitoria ternatea; Non-enzymic antioxidants; goat liver slices; H2O2.
Download this article as:| Copy the following to cite this article: Jayachitra A, Padma P. R. Non- Enzymic Antioxidant Activity of Clitoria Ternatea Leaf Extracts in Vitro. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Jayachitra A, Padma P. R. Non- Enzymic Antioxidant Activity of Clitoria Ternatea Leaf Extracts in Vitro. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9252 |
Introduction
Traditional herbal remedies are used as alternative medicine by a large proportion of people worldwide. Plants are the source of medication for preventive, curative, protective or promotive purposes (Sidhu et al., 2007). Antioxidants act as a defense mechanism that protects against oxidative damage, and include compounds and repair enzymes to remove or repair damaged molecules. However, the natural antioxidant compounds become important (Malpure et al., 2006). Antioxidants can prevent/retard the oxidation caused by free radicals and sufficient intake of antioxidants is supposed to protect against diseases (Celiktar et al., 2007). These antioxidants include (endogenous) enzymic and the (predominantly exogenous) non-enzymic antioxidants. Medicinal plants are considered as potential sources of antioxidant compounds. There is an increasing interest in the investigation of naturally occurring antioxidants from plants (Nickavar et al., 2006). One of the plants that deserves attention is Clitoria ternatea (Sanskrit-Sankupushpam) belongs to the family Fabaceae, is widely used in traditional Indian system of medicine as a brain tonic (Gomez and Kalamani, 2003).Clitoria ternatea is a perennial twinning herb bearing blue or white flowers as shown in Figure 1 .The present study is the new one concentrating on Non-enzymic antioxidant activity was analyze in both blue and white flowered leaf of Clitoria ternatea leaf extracts and also analyzed by using (goat liver slices presence and absence of oxidant H2O2 ) in vitro model.
Materials and Methods
Non-Enzymic Antioxidants in Clitoria Ternatea Leaves
The non-enzymic antioxidants analyzed in the Clitoria ternatea leaves were ascorbic acid, α-tocopherol, total carotenoids, total phenols, flavonoids, reduced glutathione and chlorophyll.
Estimation of Ascorbic Acid
Ascorbic acid, a scavenger of oxyradicals was estimated by the method of Roe and Keuther (1943). An accurate amount (1g) of leaves were homogenized in 4% TCA and made upto 10ml. Centrifuged and the supernatant obtained was treated with a pinch of activated charcoal, mixed vigorously and kept for 10 minutes. Centrifuged again to remove the charcoal residue and the supernatant obtained was used for the estimation.
Aliquots of 0.5 – 1.0ml of this supernatant were taken and 0.2 to 1.0ml of standard ascorbate were made up to 2.0ml with 4% TCA. 0.5ml of DNPH reagent was added to all the tubes, followed by 2 drops of 10% thiourea solution. The contents were mixed and incubated at 37˚C for 3 hours. The osazones formed were dissolved in 2.5ml of 85% sulphuric acid, in cold. To the blank alone, DNPH reagent and thiourea were added after the addition of sulphuric acid. After incubation for 30 minutes at room temperature, the absorbance was read spectrophotometrically at 540nm. From the standard curve constructed on an electronic calculator set to the linear regression mode, the concentration of ascorbate in the samples were calculated and expressed as mg ascorbate/g leaf.
Estimation of Tocopherol
The method described by Rosenberg (1992) was followed for the estimation of tocopherol. An exact amount (2.5g) of the homogenized plant tissue (with 5ml 0.1N sulphuric acid) was allowed to stand overnight. Then the contents of the flask were shaken vigorously and filtered through Whatmann No.1 filter paper. Aliquots of the filtrate were used for the estimation. Into 3 stoppered centrifuge tubes, 1.5ml of plant tissue extract, 1.5ml of the standard and 1.5ml of water were pipetted out respectively. To all the tubes, 1.5ml of ethanol and 1.5ml of xylene were added, mixed well and centrifuged. 0.1ml of the xylene layer was transferred into another stoppered tube and 0.1ml of 2,2’-dipyridyl reagent was added to each tube and mixed. Pipetted out 1.5ml of the mixture into a spectrophotometer cuvette and the extinction was read at 460nm. 0.33ml of ferric chloride solution was added and mixed well, and after exactly 15 minutes, the absorbance of the red colour produced was read against a blank at 520nm. The concentration of tocopherol in the sample was calculated using the formula,
The results were expressed as µg tocopherol / g leaf.
Estimation of Total Carotenoids
The method described by Zakaria et al. (1979) was followed for the estimation of total carotenoids. An exact amount (0.5g) of the sample was homogenized and saponified for about 30 minutes in a shaking water bath at 37˚C with a specific volume of 12% alcoholic KOH. The saponified extract was transferred into a separating funnel containing 10 to 15ml of petroleum ether (40-60˚C) and mixed well. The lower aqueous phase was transferred to another separating funnel and collected the upper petroleum ether containing the carotenoid pigment. The extraction was repeated until the aqueous phase was colourless. To the petroleum ether extract, a small quantity of anhydrous sodium sulphate was added to remove turbidity. The absorbance of the extract at 450nm was noted in a spectrophotometer using petroleum ether as blank.
The amount of total carotenoids was calculated using the formula,

The total carotenoids were expressed as mg/g leaf.
Estimation of Total Phenols
Total phenols were assayed by the method proposed by Mallick and Singh (1980) in plant tissue. An accurate amount (0.5g) of the leaves were homogenized in 10X volume of 80% ethanol. The homogenate was centrifuged at 10,000 rpm for 20 minutes. The residue was reextracted with 80% ethanol. The supernatants were centrifuged, pooled and evaporated to dryness. The residue was dissolved in a known volume of distilled water and 0.5ml of Folin-Ciocalteau reagent was added to it. After 3 minutes, 2.0ml of 20% sodium carbonate solution was added, mixed thoroughly and placed in a boiling water bath for exactly 1 minute, cooled and measured the absorbance at 650 nm in a spectrophotometer. Standard catechol solution (0.2-1ml) corresponding to 2.0-10 µg concentrations were added with Folin-Ciocalteau reagent and sodium carbonate. A standard curve was constructed using an electronic calculator on the linear regression mode, using which the concentrations of phenols in the samples were read. The values are expressed as mg phenols/g leaf.
Estimation of Flavonoids
Flavonoids were extracted and estimated by the method of Cameron et al. (1943).Leaves (0.5g) were extracted first with MeOH:H2O (2:1) and secondly with MeOH:H2O (1:1). The two extracts were then combined and evaporated to about 1/3 of the original volume or until most of the MeOH had been removed. The resultant aqueous extract was cleared of low polarity contaminants such as fats, terpenes, chlorophylls and xanthophylls by extraction with hexane or chloroform. This was repeated several times and the extracts combined. The solvent extracted aqueous layer containing the bulk of the flavonoids was then concentrated and used for the assay. For estimation an aliquot of the extract was pipetted out and evaporated to dryness. 4.0ml of vanillin reagent was added and heated for 15 minutes in a boiling water bath. The standard was also treated in the same manner. The optical density was read at 340nm. The values are expressed as mg flavonoids/g leaf.
Estimation of Reduced Glutathione
Estimation of reduced glutathione was done according to the procedure described by Moron et al. (1979). An exact amount (0.5g) of the plant sample was homogenized with 2.5ml of 5% TCA. The precipitated protein was centrifuged at 1000 rpm for 10 minutes. 0.1 ml of the supernatant was taken for the estimation. For estimation an accurate aliquot (0.1 ml) of the supernatant was made up to 1.0 ml with 0.2M sodium phosphate buffer. Freshly prepared DTNB solution (2.0 ml) was added and the intensity of the yellow colour formed was read at 412nm in a spectrophotometer after 10 minutes. A standard curve of GSH was prepared between the concentration ranges of 2 to 10 nmoles. The values are expressed as nmoles GSH/g leaf.
Estimation of Chlorophyll
The estimation of chlorophyll was done according to the procedure described by Witham et al. (1971). An accurate amount (1g) of leaves were extracted with 20ml of 80% acetone, centrifuged (5000rpm for 5 minutes) and transferred the supernatant to a 100ml volumetric flask. This procedure was repeated until the residue was colourless. The supernatant was made upto 100 ml with 80% acetone. The absorbance of the solution was read at 645nm and 663nm against 80% acetone blank. The amount of chlorophyll present in the extract was calculated using the following formula:
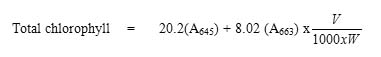
where V was the final volume of the extract and W was the fresh weight of the leaves taken for extraction. The results are expressed as mg chlorophyll/g leaf.
Antioxidant Status in Vitro
In vitro testing encompasses the use of ultra sensitive probes to test (or to study) the single effect or action of a substance in an isolated environment eliminating interference from other biological phenomena which contribute to a reduction in the number of animals (Robinson, 1991). The in vitro model used in the study as alternatives to live animals was goat liver slices. The effect of the exposure of the leaf extracts in the presence or absence of the oxidants was followed in precision-cut goat liver slices, which simulated the in vivo environment.
Fresh goat liver was obtained from a local slaughter house and transported to the laboratory on ice. The liver was washed with isotonic KCl and processed for the assays. Liver was the organ of choice because it is the metabolic organ and is responsible for the metabolic clearance of many xenobiotics (Tingle and Helsby, 2006). Very thin slices (~1mm thick) were cut from the liver using a sterile scalpel. The slices were taken in sterile Hank’s balanced salt solution (HBSS) at a proportion of 0.25g in 1ml, in broad, flat-bottomed flasks. The standard oxidant H2O2 was used at a final concentration of 200µM. The plant extract (20 µl of the plant extract corresponding to 20mg) was used to study the antioxidant effect on the cells. The liver slices exposed to different treatments were incubated for one hour at 37˚C with very mild shaking.
Untreated (negative) control
H2O2 treated (positive) control
Group treated with white flowered leaf extract
Group treated H2O2 and white flowered leaf extract
Group treated with blue flowered leaf extract
Group treated with H2O2 and blue flowered leaf extract
After the addition of the respective agents, the tissue slices were incubated at 37˚C for one hour with mild shaking. After the incubation period, the tissue was homogenized in a Teflon homogenizer with HBSS. The estimation of various parameters indicative of antioxidant potential were carried out in the homogenate as explained below.
Effect of Leaf Extract Treatment on the Levels of Non-Enzymic Antioxidants in Liver Slices Subjected to Oxidant Stress
The non-enzymic antioxidants determined in the liver slice homogenate were ascorbic acid, tocopherol, vitamin A and reduced glutathione. The procedures adopted for the determination of vitamin C, vitamin E and reduced glutathione were the same as those for Clitoria ternatea leaf analysis. An aliquot of the tissue homogenate was used instead of leaf tissue in the assay.
Estimation of Vitamin A
Vitamin A was estimated by the method of Bayfield and Cole (1980). To 1.0ml homogenate, 1ml of saponification mixture was added. The tubes were gently refluxed for 20 minutes at 60˚C. The tubes were cooled at room temperature, 20ml of water was added and mixed well. Vitamin A was extracted twice with 10ml portions of petroleum ether (40-60˚). The extracts were pooled, washed thoroughly with water, separating the layers using a separating funnel. When the petroleum ether fraction was clear, a pinch of sodium sulphate (anhydrous) was added to remove the excess moisture. The volume of the extract was noted and 1.0ml of it was evaporated to dryness at 60˚C. The dried residue was dissolved in 1.0ml of chloroform. Aliquots of the standard were pipetted out into a series of clean, dry test tubes in the concentration range of 0-7.5 µg. The volumes in all the test tubes were made up to 1.0ml with chloroform. From a fast delivery pipette, 2ml of TCA reagent was added rapidly, mixing with the contents of the tube. The absorbance was recorded immediately at 620nm in a spectrophotometer. The procedure was repeated for the sample tubes. A standard graph was constructed on an electronic calculator set to the linear regression mode, using which the concentration in the samples were read off and expressed as µg/g tissue.
Results
Levels of Non-Enzymic Antioxidants in Clitoria Ternatea Leaves
The major representatives of the non-enzymic antioxidants, namely ascorbate, tocopherol, total carotenoids, reduced glutathione, total phenols, flavonoids and total chlorophyll were estimated in blue and white flowered leaves. The concentrations of non-enzymic antioxidants observed in the leaves of Clitoria ternatea are presented in Table 1.
Table 1: Levels Of Non-Enzymic Antioxidants In Clitoria Ternatea Leaves
| Parameter | Blue flowered leaf | White flowered leaf |
| Ascorbic acid (mg/g) | 3.91 ± 0.032 | 5.56 ± 0.015a |
| Tocopherol (mg/g) | 4.73 ± 0.126 | 8.55 ± 0.057a |
| Total carotenoids (mg/g) | 8.65 ± 0.086 | 9.54 ± 0.061a |
| Reducedglutathione
(nmoles/g) |
33.00 ± 1.000 | 39.00 ± 1.000a |
| Total phenol (mg/g) | 13.59 ± 0.537 | 16.30 ± 0.304a |
| Flavonoids (mg/g) | 16.00 ± 0.577 | 24.00 ± 0.579a |
| Total chlorophyll (mg/g) | 1.18 ± 0.020 | 1.37 ± 0.025a |
Values are mean ± SD of triplicates.
a – Statistically significant (P< 0.05) compared to blue flowered leaves.
From these results, it can be observed that both the varieties of leaves are good sources of non-enzymic antioxidants. The white flowered leaves had higher content of all the non-enzymic antioxidants analyzed than the blue flowered ones.
Effect of Clitoria Ternatea Leaves on the Antioxidant Status of Cells Exposed to Oxidative Stress in Vitro
To minimize the number of animals needed for testing, alternative methods, such as in vitro and computer technology, and use of non-mammalian organisms, plays an increasingly important role in research, which incorporates reduction, refinement and replacement of animals for a particular study (Goldberg and Hartung, 2006). The standard oxidant used to induce the oxidative stress was H2O2. The activities of non-enzymic antioxidants were recorded in precision-cut goat liver slices exposed to the standard oxidant (H2O2) in the presence and the absence of ethanolic leaf extracts of Clitoria ternatea. Following a one hour exposure, the non-enzymic antioxidants were estimated in the tissue slices.
Ascorbic Acid
The levels of vitamin C observed in the different treatment groups are presented in Table 2. The levels of vitamin C in the liver slices increased significantly upon exposure to the leaf extracts alone, in the absence of oxidative stress. When the slices were stressed with H2O2, the vitamin C levels depleted significantly (P<0.05). The effects of the leaves on vitamin C levels were more pronounced in the slices treated with the white flowered leaves than the blue flowered leaves.
Table 2: Effect of Clitoria Ternatea Leaves on the Levels of Vitamin C in H2o2 – Induced Oxidative Stress in Goat Liver Slices
| Sample | Vitamin C Levels (mg/g tissue) | |
| Without H2O2 | With H2O2 | |
| Control | 0.133 ± 0.020 | 0.127 ± 0.004 a |
| Blue flowered leaf | 0.301 ± 0.020 a | 0.147 ± 0.004 a b c |
| White flowered leaf | 0.890 ± 0.036 a | 0.264 ± 0.02 a b c |
LSD (5%) = 0.034
Values are mean ± SD (n = 3)
a – Statistically significant (P<0.05) compared to untreated control group.
b – Statistically significant (P<0.05) compared to H2O2 treated group.
c – Statistically significant (P<0.05) compared to respective plant group.
Vitamin E
Vitamin E, a major lipid soluble antioxidant, is the most effective chain breaking antioxidant within the cell membrane, where it protects membrane lipids from lipid peroxidation .The levels of vitamin E observed in the various treatment groups are listed in Table 3. The levels of vitamin E in the liver slices after exposure to the leaf extracts showed a significant increase when compared to the untreated group. The oxidant treatment decreased the levels of vitamin E compared to untreated control. This depletion was counteracted by the co-treatment with the leaf extracts. The levels were found to be higher in the white flowered leaf treated group, than the blue one .
Table 3: Effect of Clitoria Ternatea Leaves on the Levels of Vitamin E in H2o2 – Induced Oxidative Stress in Goat Liver Slices
| Sample | Vitamin E Levels (mg/g tissue) | |
| Without H2O2 | With H2O2 | |
| Control | 1.20 ± 0.05 | 1.05 ± 0.08 a |
| Blue flowered leaf | 2.40 ± 0.13 a | 1.95 ± 0.89 a b c |
| White flowered leaf | 2.95 ± 0.07 a | 2.25 ± 0.05 a b c |
LSD (5%) = 0.632
Values are mean ± SD (n = 3)
a – Statistically significant (P<0.05) compared to untreated control group.
b – Statistically significant (P<0.05) compared to H2O2 treated group.
c – Statistically significant (P<0.05) compared to respective plant group.
Vitamin A
The levels of vitamin A in the goat liver slices exposed to H2O2 in the presence and absence of Clitoria ternatea leaf extracts are represented in Table 4. Among the two, the liver slices treated with the white flowered leaves showed significant increase in vitamin A levels than the ones treated with blue flowered leaves.
Table 4: Effect of Clitoria Ternatea Leaves on the Levels of Vitamin A in H2o2 – Induced Oxidative Stress in Goat Liver Slices
| Sample | Vitamin A Levels (mg/g tissue) | |
| Without H2O2 | With H2O2 | |
| Control | 43.83 ±0.021 | 37.00 ± 1.00 a |
| Blue flowered leaf | 62.07 ± 0.90 a | 46.27 ± 1.10a b c |
| White flowered leaf | 71.13 ± 1.03 a | 55.59 ± 0.55a b c |
LSD (5%) = 1.528
Values are mean ± SD (n=3)
a – Statistically significant (P<0.05) compared to untreated control group.
b – Significant (P<0.05) compared to H2O2 treated group.
c – Statistically significant (P<0.05) compared to respective plant group.
Reduced Glutathione (Gsh)
Reduced glutathione levels were estimated in the liver slices after quick acidification of the reaction mixture to prevent aerial oxidation of the compound. The levels of the same are listed in Table 5. Once again the results showed that the impact of white flowered leaves was higher than that of blue flowered leaves
Table 5: Effect o0f Clitoria Ternatea Leaves on the Levels of Reduced Glutathione in H2o2 – Induced Oxidative Stress inGoat Liver Slices
| Sample | GSH Levels (nmoles/g tissue) | |
| Without H2O2 | With H2O2 | |
| Control | 266.13 ± 1.40 | 246.40 ± 0.31 a |
| Blue flowered leaf | 384.53 ± 0.21 a | 269.33 ± 0.16a b c |
| White flowered leaf | 481.30 ± 1.70a | 362.13 ± 0.55 a b c |
LSD (5%) = 0.727
Values are mean ± SD (n = 3)
a – Statistically significant (P<0.05) compared to untreatedcontrol group.
b – Statistically significant (P<0.05) compared to H2O2 treated group.
c – Statistically significant (P<0.05) compared to respective plant group.
 |
Figure 1
|
Discussion
Non-Enzymic Antioxidants in Clitoria Ternatea Leaves
Cervantes et al. (2007) have found that the aqueous extracts of Cissus quadrangularis showed high levels of ascorbic acid. These antioxidant properties were also shown to depend on the concentration of the sample (Serteser et al., 2008). Different varieties of Cyanora scolymus (Artichoke) exhibited differences in polyphenolic content (Wang et al., 2003). Gladiolus tepal flower senescence showed decreased level of ascorbate and peroxidase activity when exposed to H2O2 oxidant (Hossain et al., 2006). In very recent studies, Zizyphus jujuba (Pawlowska et al., 2009), Teucrium polium. L (Sharfifar et al., 2009), Whilst rush crimps (Conforti et al., 2009) and raspberries (Wange et al., 2009) have been shown to possess higher level of phenols and flavonoid content. The results showed that the white flowered leaves possessed higher levels of the non-enzymic antioxidants and revealed that the white flowered leaves of Clitoria ternatea showed the maximum antioxidant content when compared to blue flowered leaves..
Non-Enzymic Antioxidants Analyzed in Liver
The non-enzymic antioxidants analyzed in the liver slices exposed in vitro to H2O2 and / or Clitoria ternatea leaf extracts were vitamins C, E, A and reduced glutathione.
Vitamin C
The level of vitamin C was significantly reduced by the treatment with H2O2. This reduction was reversed by the presence of the leaf extracts of Clitoria ternatea. Ascorbic acid is a terminal water soluble antioxidant that protects lipids against peroxidation (Maneesh et al., 2005). It readily oxidizes the dehydro ascorbic acid and interferes with the process of LPO by scavenging the superoxide anion (Jariyapongskul et al., 2002). Vitamin C supplementation in humans increases plasma ascorbate and improves the resistance of plasma lipids to LPO (Polidori et al., 2004, Linster and Van Schaftingen, 2007). Vitamin C is required for the optimal activity of important biosynthetic enzymes and is therefore essential for various metabolic pathways in the body (Carr and Frei, 1999). Thus the effect was more pronounced in the slices treated with the white flowered leaves than the blue flowered leaves.
Vitamin E
The present study may be due to the consumption of vitamin E to quench the free radical generated by H2O2. The levels of vitamin E were restored by the ethanolic extracts of Clitoria ternatea leaves. Vitamin E, a major lipid soluble antioxidant, is the most effective in preventing chain breaking within cell membrane, where it protects membrane fatty acids from lipid peroxidation (Brown and Goodman, 1998). Hippophea rhamnoides improved the levels of vitamin E to overcome the oxidative stress induced by nicotine in rats (Gumustekin et al., 2003). Thus Clitoria ternatea leaf extracts can render protection to the membranes by increasing the levels of vitamin E, the major antioxidant present in the membrane.
Vitamin A
Retinol and retinyl esters are precursors of retinoic acid, the most active form of vitamin A and a ligand for retinoid receptors. Retinoic acid plays an important role in controlling cell growth, cell differentiation and apoptosis as well as carcinogenesis, and is of potential clinical interest in cancer chemoprevention and treatment (Altucci and Gronemeyer, 2001). Carotenoids, such as a-carotene, b-carotene, lycopene, lutein and cryptoxanthine, have been shown to have antioxidant properties (Donaldson, 2004). Vitamin A also prevents hepatic injury caused by H2O2 treatment (Noyan et al., 2006). There was a reduction in plasma vitamin A during oxidant intoxication both in a-tocopherol acetate supplemented and unsupplemented humans (MacDonald-Wicks and Garg, 2003). Clitoria ternatea leaf extracts very effectively increased the levels, reiterating the antioxidant effect of the leaf extracts. Among the two leaf extracts, the white flowered leaf extract caused a more significant increase in vitamin A levels in the liver slices than the blue flowered leaves.
Reduced Glutathione
GSH maintains functional and structural integrity of cells and is often involved in the defense against tissue injury from administered or metabolically generated toxic agents (Ortman et al., 2000). Reduced glutathione levels decreased in alloxan treated rats, which was efficiently counteracted by the administration of Boerhavia diffusa leaf extracts (Satheesh and Pari, 2004). Administration of the flower extract of Punica granutum (pomegranate) (Kaur et al., 2006), melatonin (Dakshayani et al., 2005) and curcumin (Marotta et al., 2003) increased the levels of glutathione which had been depleted by severe liver injury in experimental rats. Thus the effect was more pronounced in the slices treated with the white flowered leaves than the blue flowered leaves.
In the present study, the use of liver slices were subjected to oxidative stress using H2O2 and the effect of the co-administration of the leaf extracts was monitored on the antioxidant status. Our results show that the Clitoria ternatea leaf extracts as reflected by the beneficial alterations in the non- enzymic antioxidants. Among the two, the white flowered leaves rendered slightly higher induction of non- enzymic antioxidants than the blue flowered leaves.
Acknowledgments
This work was supported by Avinashilingam university,Coimbatore-43
References
- Altucci, L. and Gronemeyer, H. (2001), The promise of retinoids to fight against cancer, Nat. Rev. Cancer, 1, 181-193.
- Bayfield, R.F. and Cole, E.R. (1980), Colorimetric estimation of vitamin A with trichloroacetic acid, Meth. Enzymol., 67, 189-195.
- Brown, D.J. and Goodman, J. (1998), A review of vitamin A, C and E and their realationship to cardiovascular disease, Clin. Excell. Nurse Pract., 2, 10-12.
- Cameron, G.R., Mitton, R.F. and Allan, J.W. (1943), Lancet, 179.
- Carr, A.C. and Frei, B. (1999), Towards a new recommended dietary allowance for vitamin C based on antioxidants and health effects in humans, Am. J. Clin. Nutr., 69, 86-107.
- Celiktar, O.Y., Girgin, G., Orhan, H., Nichers, H.J., Bedir, E. and Sukan, F.V. (2007), Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus officinalis extracts with focus on location and harvesting times, Eur. Food Res. Technol., 24, 443-451.
- Cervantes, H., Jainu, M. and Devi, S.C.S. (2007), In vitro evaulation of the free radical scavenging potential of Cissus quadrangulairs, Afr. J. Biomed. Res., 8, 95-99.
- Conforti, F., Sosa, S., Marrelli, M., Menichini, F., Statti, G.A., Uzunov. D., Tubaro. A. and Menichini, F. (2009), The protective ability of Mediterranean dietary plants against the oxidative damage. The role of radical oxygen species in inflammation and the polyphenol, Haronoid and sterol contents, Food. Chem., 112, 587-594.
- Dakshayani, K.B., Subramanian, P., Manivasagam, T., Essa, M.M. and Manoharan, S. (2005), Melatonin modulates the oxidant-antioxidant imbalance during N-nitrodiethylamine induced hepatocarcinogenesis in rats, J. Pharm. Spinal Cord Injury, 8, 316-321.
- Gomez, S.M. and Kalamani, A. (2003), Butterfly pea (Clitoria ternatea): A nutritive multipurpose forage legume for the tropics–An Overview, Pak. J. Nutr., 2, 374-379.
- Goldberg, A.M. and Hartung, T. (2006), Protecting more than animals, Sci. Am, India, 1, 66-73.
- Gumustekin, K., Altinkayanak, K., Timur, H., Taysis, S., Oztasan, N., Pelat, M. E., Akcay, F., Suleyan, H., Dane, S. and Gul, M. (2003), Vitamian E and Hipophea rhamnoides .L prevented nicotine-induced oxidative stress in rat brain, Hum. Exp.Toxicol., 22, 425-431.
- Hossain, Z., Manda, A.K., Datta, K. S. and Biswas, K. A. (2006), Decline in ascorbate peroxidase activity-A prerequisite factor for Gladiolus tepal senenscence, J. Plant, Phsiol., 163, 186-194.
- Jariya-Pongskul, A., Patumraj, S., Yamaguchi, S. and Nimi, H. (2002), The effect of longterm supplementation of vitamin C on leukocyte adhesion to the cerebral endothelium in STZ induced diabetic rats, Clinical Hemorheol. Microcirc., 27, 67-76.
- Kaur, G., Jabbar, Z. Athar, M. and Alam, M.S. (2006), Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and check Fe-NTA induced hepatotoxicity in mice, Food. Chem. Toxicol., 40,12-20.
- Linster, C.L. and Van Schaftingen, E. (2007), Vitamin C, Febs. J., 274, 1-22.
- Mac Donald-Wicks, L.K. and Garg, M.L. (2003), Vitamin E supplementation in the mitigation of carbon tetrachloride – induced oxidative stress in rats, J. Nutr. Biochem., 14, 211-218
- Mallick, C. P. and Singh, M. B. (1980), Plant enzymology and plant histoenzymology, Kalaya. Pub., New. Delhi., 281- 286.
- Malpure, P.P., Shah, A.S. and Juvekar, A.R. (2006), Antioxidant and anti-inflammatory activity of extract obtained from Aspergillus candidus MTCC 2202 broth filterate, Ind. J. Exp. Biol., 44, 468-473.
- Marotta, F., Shieldm Y.R., Bambam T., Naito, V., Minell, E. and Xoshioka, M. (2003), Hepatoprotective effect of a Curcumin labsinthium compound in experimental severe liver injury, Chin. J. Digest. Dise., 4, 122-126.
- Maneesh, M., Jayalakshmi, H., Dutta, S., Chakrabarti, A. and Vasudevan, D.M. (2005), Experimental therapeutic intervention with ascorbic acid in ethanol induced testicular injuries in rats, Ind. J. Exp. Biol., 43, 172-176.
- Moron, M.S., Depierre, J.N. and Mannervik, Y.C. (1979), Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver, Biochem. Biophys. Acta., 582, 67-68.
- Nickavar, B., Kamalinelad, I., Yahya, M.H. and Shalaghi, B. (2006), Comparison of the free radical scavenging activity of six Iranian Achillea species, Pharm.Biol., 44, 208-212.
- Ortman, J.K., Durinska, M., Frandin, M. and Somoro, V. and Ka, M. (2000), Assay of glutathione, Glutathione disulphide and glutathione mixed disulphide in biological samples, Meth. Enzymol., 77, 373-382.
- Polidori, M.C., McCocci, P., Levine, M. and Frei, B. (2004), Short term and long term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex – vivo lipid peroxidation, Arch. Biochem. Biophys., 43, 109-115.
- Roe, J. H. and Keuther, C. A. (1943), The determination of ascorbic acid in whole blood and urine through 2, 4-dinitro phenyl hydrazine derivative of dehydro ascorbic acid, J. Biol. Chem., 147, 399- 407.
- Rosenberg, H.R. (1992), Chemistry and physiology of the vitamins, Intersci. Pub, N.Y., 452-453.
- Robinson, C.J. (1991), Meeting report of cell-based assays technology providing alternatives to animal testing, TIBTech., 9, 407-408.
- Satheesh, A.M. and Pari, L. (2004), Antioxidant effect of Boerhavia diffusa L. in tissues of alloxan induced diabetic rats, Ind. J. Exp. Biol., 42, 989-992.
- Serteser, A., Kargioglu, M., Gok, V., Bagci, Y., Ozcan, M.M. and Arslan, D. (2008), Determination of antioxidant effects of some plant species wild growing in Turkey, Int. J. Food. Sci. Nutr., 12, 1-9.
- Sharfifar, F., Mudeh.G. D. and Mirtajaldini. M., (2009), Major flavonoids with antioxidant activity from Teucrium polium.L. Food, Chem., 112, 4,885-888.
- Sidhu, K., Kaur, J., Kaur, G. and Pannu, K. (2007), Prevention and cure of digestive disorders through the use of medicinal plants, J. Hum. Ecol., 21, 113-116.
- Tingle, M.D. and Helsby, N.A. (2006), Can in vitro drug metabolism studies with human tissues replace in vitro animal studies? Environ.Toxicol. Pharmacol., 21, 184-190.
- Wang, L., Yen, J.H., Liang, H.L. and Wu, M.J. (2003), Antioxidant effect of methanol extracts from lotus plumule (Nelumbo nucifera) and blossom, Ind. J. Biochem. Biophys., 38, 321-326.
- Wange, Y.S. Chen, C.T., Wang, C.Y. (2009), The influence of light and maturity on fruit quality and flavonoid content of red raspberries, Food. Chem. 112, 676-684.
- Witham, F.H., Blaydes, B.F. and Devlin, R.M. (1971), Experiments in plant physiology., Van. Nos. N. Y., P.245.
- Zakaria, H., Simpson, K., Brown, P. R. and Krotulovic, A. (1979), Use of reversed phase HPLC analysis for the determination of provitamin A carotenes in tomatoes, J. Chrom, 176, 109- 117.

This work is licensed under a Creative Commons Attribution 4.0 International License.






