How to Cite | Publication History | PlumX Article Matrix
Fawkeya Abd Alla Abbas
Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Zagazig - 44519 Egypt.
Corresponding Author E-mail:fawkeya@yahoo.com
ABSTRACT: Repeated column chromatography of the butanol extract of Scrophularia xanthoglossa yielded three phenylpropanoid glycosides identified as scropheanoside-I, scropheanoside-II, scropheanoside-III together with two phenylethanoid glycosides identified as, acetoside, and martynoside. Their structures were determined by spectroscopic analysis, chemical evidence as well as comparison with literature values. The biological activity was also studied where acetoside and martynoside showed significant antioxidative activity, while scropheanoside-III showed significant anti-inflammatory activity.
KEYWORDS: Scrophularia xanthoglossa; Scrophulariaceae; Scropheanosides I-III
Download this article as:| Copy the following to cite this article: Alla-Abbas F. A. Phenylpropanoid and Phenylethanoid Glycosides from Scrophularia Xanthoglossa and Their Antioxidative and Antiinflammatory Activities.Biosci Biotechnol Res Asia 2010;7(1) |
| Copy the following to cite this URL: Alla-Abbas F. A. Phenylpropanoid and Phenylethanoid Glycosides from Scrophularia Xanthoglossa and Their Antioxidative and Antiinflammatory Activities.Biosci Biotechnol Res Asia 2010;7(1).Available from: https://www.biotech-asia.org/?p=8863 |
Introduction
In our previous study [Abbas and Zayed, 2004], we reported the isolation and structure determination of five iridoid glycosides; aucubin, harpagid, 6-O-α-L-rhamnopyranosyl-aucubin, harpagoside and 6-O-α-L-rhamnopyranosyl-catalpol from Scrophularia xanthoglossa Bioss. A survey of literature showed that no phytochemical and pharmacological works have been reported on phenylpropanoid and phenylethanoid glycosides from Scrophularia xanthoglossa Bioss., a perennial plant growing in Yemen and southwestern Arabia [Miagahid,1989]. In continuation to our studies on the constituents of Scrophularia xanthoglossa, we investigated the butanol extract of the plant to furnish the isolation and identification of three phenylpropanoid glycosides scropheanosides I-III, and two phenylethanoid glycosides; acetoside and martynoside. The antioxidative activity of the five glycosides was evaluated against 2, 2- diphenyl-1-picrylhydrazyl (DPPH). The five compounds isolated were also evaluated as anti-inflammatory, where scropheanoside-III was the most active among all compounds tested.
Experimental
General Experimental Procedures
Optical rotation were measured on a Zeiss polarimeter Model 53187 using a sodium lamp. UV spectra were obtained on a Varian CARY 2290 spectrophotometer in Me OH. IR spectra were recorded on a Perkin –Elmer Model 337 spectrometer in K Br discs. FABMS were recorded with an MS 2500 high-resolution spectrometer ( Kratos , Manchester, UK) with 70 eV, an ion source temperature of 180 o C and a direct inlet using meta nitrobenzyl alcohol and/ or thioglycerin as matrix. NMR spectra were measured on CD3OD and pyridine-d5 and recorded at 300 MHz for 1 H-NMR and 75 MHz for 13C-NMR using TMS as internal standard on a Varian XL300 (Darmstadt, Germany ). Chemical shifts were expressed in δ values (1H) and ppm (13C-NMR). Precoated thin–layer chromatographic (TLC) plates coated with silica gel 60 F254 ,0.25 mm layer thickness and silica gel ( 60-120 mesh ) for column chromatography were obtained from Merck ( Darmstadt, Germany ). The spots were visualized by spraying with 1% vanillin-H2SO4 solution followed by heating at 100 o C for 10 minutes. Solvent systems used for TLC were: I. CHCl3-CH3OH-H2O (15:7.5:2.5, lower phase); II. Et OAc – CH3OH – n-C3H7OH – H2O (15:10:2:7, lower phase ).
Plant Material
Scrophularia xanthoglossa Bioss. is a glabrous perennial plant ( up to one meter high ) that grows in Yemen and Southwestern Arabia [Miagahid,1989]. The sample of aerial parts for the present study was collected from road and hill sides in Sanaa region (Wadi Zahr) in Yemen in March 1997. Identity of the plant was confirmed by Professor Sultan Abdeen, Faculty of Pharmacy, King Saud University. A certified specimen has been deposited at the Pharmacognosy Department, Faculty of Pharmacy, King Saud University, Saudi Arabia. The aerial parts of the plant were air-dried and powdered before extraction.
Extraction and Isolation
The air-dried powdered plant material of Scrophularia xanthoglossa Bioss. (500g) was exhaustively extracted with ethanol (95%, 5 x 2 L) by cold maceration. The solvent was distilled off under reduced pressure, then the residue (60 g) was suspended in distilled water (500ml), the water-insoluble material was removed by filtration. The aqueous solution was successively extracted with petroleum ether, ether, chloroform, ethyl acetate, and finally with butanol saturated with water (3 L each ) .The solvent in each case was distilled off under reduced pressure to afford ( 13 , 7, 2 , 11, 17 g , respectively ) . TLC examination of the butanol extract using solvent system II revealed the presence of five spots having R f s 0.56, 0.51, 0.47, 0.36.and 0.31.
About 10 g of the dried butanol extract were subjected to flash silica gel column (Merck, 500 g, 4.5×120 cm ) using CHCl3 – MeOH –H2O gradient elution and repeated purification columns to afford the following compounds:
Compound 1
Obtained as a white amorphous powder; [α]D25, -100O (CH3OH, c =1 ) with R f s 0.51 (system I ) and 0.56 (system II). IR ( K Br ) : √ max cm-1 3400, 2900, 1700, 1660, 1630, 1580, 1510, 1440, 1260, 1030. Positive FABMS: m/z 691 [M +Na ]+, 669 [ M +H ]+ , 323 [ C16H18O7 ]+, 177 [C10H9O3]+.1H-NMR (300 MHz, CD3OD, δ , ppm, J= Hz ): 4.9 (d, J=7 Hz, 1- H ), 6.3 (dd, J=6.5 & 2 Hz, 3-H ), 5.1 (dd, J=6.5 & 4 Hz, 4-H ), 2.8 (dddd, J =7.5, 5.5 , 4 & 2 Hz, 5- H ), 4.4 (m ,6- H ), 5.7 (dddd, J =2,2,2&2 Hz, 7-H ), 2.88 (dd, J=7.5 & 7 Hz, 9-H ), 4.23 (d, J=16 Hz , 10 a -H), 4.34 ( d, J= 16 Hz, 10 b -H), 4.7 (d, J=8 Hz, 1′-H), 3.2 ( dd, J=9 & 8 Hz, 2′-H ), 3.4 (dd, J= 9 & 9 Hz , 3′-H), 3.26 – 3. 34 (m, 4′-H ) , 3.26-3.34 (m, 5′-H), 3.6 (dd, J= 12 & 6 Hz, 6′a– H ), 3.8 (dd, J= 12 & 2 Hz, 6′b-H ), 4.8 (d, J=2 Hz, 1″-H ), 3.83 (dd , J =2 & 3.5 Hz , 2″-H ), 3.86 (dd, J =9.5 & 3.5 Hz , 3″-H ), 5 (d, J=9.5 Hz, 4″-H), 3.9 (dd, J = 9. 5 & 6.5 Hz, 5″-H ), 1.2 ( d, J =6.5 Hz , 6″-H ), 7.09 (d, J =2 Hz, 2′”-H ), 6.9 (d, J= 8 Hz, 5″‘-H ), 7.06 (dd, J = 8 & 2 Hz, 6″‘-H ), 6.35 (d, J =16 Hz, α-H ), 7.6 (d, J= 16 Hz, β-H ), 3.8 (s, OCH3). 13C-NMR (75 MHz, CD3OD , δ , ppm) see Table 1.
Compound 2
Obtained as white amorphous powder; [α]D 25 -1800 (CH3OH, c=1) with R f s 0.47 ( system I) and 0.51 (system II ). IR ( KBr ) : √ max cm-1 3400, 2900, 1630, 1610, 1600, 1580, 1500. Positive FABMS : m/z 685 [M+H]+ Calcd for the formula (C31H40O17+H ), 707 [M+Na]+, 323 , 177. 1H-NMR (300 MHz, CD3OD, δ , ppm, J =Hz ): 5.1 (d, J=9.5 Hz,1-H), 6.37 (dd, J=6.5 &2 Hz, 3-H), 5 (dd, J=6.5 &4 Hz, 4-H), 2.4 (dddd, J=8,8,4&2 Hz, 5-H), 4 (dd, J=8 &2 Hz, 6-H) , 3.7 (d, J=2 Hz ,7-H), 2.7 (dd J=8 &9.5 Hz, 9-H), 3.8 (d, J=13 Hz, 10a-H), 4.2 (d, J=13 Hz, 10b-H), 4.8 (d, J=8 Hz, 1′-H), 3.3 (dd, J=9 &8 Hz, 2′-H), 3.4 (dd, J=9 &9 Hz,3′-H), 3.2 (dd, J=9 &8 Hz, 4′-H), 3.3 (m, 5′-H), 3.6 (dd, J= 6 &12 Hz, 6′a-H), 3.9 (dd, J=12 &6 Hz, 6′b-H), 5 (d, J= 2 Hz, 1″-H), 3.9 (dd, J= 4 & 2 Hz, 2″-H ), 3.8 (dd, J=9.5 &4 Hz, 3″H), 5 (dd, J=9.5 &9.5 Hz, 4″-H), 3.89 (dd, J= 6 &9.5 Hz,5″-H), 1.2 (d, J= 6 Hz, 6″-H ), 7 (d, J=2 Hz, 2″‘H), 6.9 (d, J= 8.5 Hz, 5″‘-H), 7 (dd, J=8.5 &2 Hz, 6″‘-H ), 6.4 (d, J=16 Hz, α- H) , 7.6 (d, J=16 Hz, β-H) ,3.9 ( s, OCH3). 13C-NMR (75 MHz, CD3OD , δ, ppm ) see Table 1.
Compound 3
Obtained as white amorphous powder; [α]D 25 -890 (CH3OH ,c=1) with R f s 0.43 (system I) and o.47 (system II ). IR ( KBr ) : √ max cm-1 3400 ,1745 , 1715 ,1630 1575 .Positive FABMS :m/z 740 [M +NH4]+, 745 [M+Na]+ Calcd for the formula (C34H42O17), 361 (M- C15H12O10) .1H-NMR (300 MHz, CD3OD , δ , ppm , J=Hz): of the catalpol nucleus and the glucose moiety are in good agreement with those of compound 2 isolated from the same plant, 5.1 (d, J=2 Hz, 1″-H) ), 5.3 (dd, J= 4 & 2 Hz, 2″-H ), 5.4 (dd, J=9.5 &4 Hz, 3″-H), 5 (dd, J=9.5 &9.5 Hz, 4″-H), 4 (dd, J= 6 &9.5 Hz, 5″-H), 1.2 (d, J= 6 Hz, 6″-H ), 7.4 (m , 2″‘-H), 7.6 (m, 3″‘H), 7.4 (m,4″‘-H), 7.6 (m, 5″‘-H) 7.6 (m, 6″‘-H), 6.4 (d , J=16 Hz, α-H), 7.6 (d, J=16 Hz, β-H) , 2 ( s, COCH3) , 2.1 (s, CO-CH3) . 13C-NMR (75 MHz, CD3OD , δ , ppm ) see Table 1.
Compound 4
Obtained as yellowish-white amorphous powder; [α]D 25 -310 (CH3OH , c=1) with R f s 0.33 (system I) and 0.36 (system II ). IR ( KBr ) : √ max cm-1 3380 , 2920 , 1795 ,1630 ,1600,1510. .Positive FABMS : m/z 625 [M +H]+, 642 [M+NH4]+, 647 [M+Na]+ Calcd for the formula ( C29H36O15). The UV (MeOH, λmax ,nm) 320, 280 and 230 nm. 1H-NMR (300 MHz, CD3OD , δ , ppm , J=Hz): 6.6 (d, J=2 Hz, 2-H) , 6.7 (d, J=8 Hz, 5-H) , 6.5 (dd, J=2 & 8Hz, 6-H) ,3.7(dd, J=7 & 17 Hz, α-Ha), 4 (dd, J=7 &17 Hz, α-Hb) ,2.8 (dd, J=7 &7, β –Ha, b ), 4.4 (d, J= 8 Hz,1″-H), 3.4 (dd, J = 8 & 9 Hz, 2″ –H ), 3.8 (d, J = 8 Hz, 3″- H ), 4.8 (m, 4″-H ), 3.6 (m, 5″-H , 6″-H a, b ), 6.3 (d, J=16 Hz, α’-H ), 7.6 (d, J= 16Hz, β’-H), 7 (d, J= 2 Hz, 2H), 6.8 (d, J=8 Hz, 5′-H ), 6.9 (dd, J= 2 &8 Hz,6′-H ), 5.2 (d, J=2 Hz, 1″‘-H ), 3.9 (dd, J= 2 &3.5 Hz, 2″‘-H ), 3.5 (dd, J= 9.5 &9.5 Hz, 3″‘-H ), 3.3 (dd, J= 9.5 &9.5 Hz, 4″‘-H ), 3.4 (m, 5″‘-H ), 1 (d, J= 6 Hz, 6″‘-H ) . 13C-NMR (75 MHz, CD3OD , δ , ppm ): 131 (C-1 ), 117 ( C-2 ), 144 (C-3 ), 146 (C-4 ), 116 (C-5 ), 121 (C-6 ), 72 (C-α ), 36 (C-β ), 104 (C-1″), 76 (C-2″), 81 (C-3″), 70 (C-4″), 76 (C-5″), 62 (C-6″), 114 (C-α’), 147 (C-β’), 168 (C=O), 127 (C-1′),115 (C-2′), 149 (C-3′), 147 (C-4′), 116 (C-5′), 123 (C-6′), 102 (C-1″‘), 72 (C-2″‘), 71 (C-3″‘), 73 (C-4″‘), 70 (C-5″‘), 18 (C-6″‘).
Compound 5
Obtained as white amorphous powder; [α]D25 -710 (CH3OH , c=1) with R f s 0.29 (system I) and o.31 (system II ). IR ( K Br ) : √ max cm-1 3400 ,2920 , 1695 ,1630 ,1600,1520. .Positive FAB MS :m/z 653 [M +H]+, 670 [M+NH4]+, Calcd for the formula (C31H 40O15). The UV (Me OH, λmax , nm) :328, 288. 1H-NMR (300 MHz, CD3OD , δ , ppm ,J=Hz) of the compound showed close similarity to the 1HNMR of compound 4 isolated from the same plant, except for the presence of two methoxyl groups [δ H 3.8 (s, 4- OCH3 ) and δ H 3.9 (s, 3′- OCH3 ) ]. 13C-NMR (75MHz, CD3OD, δ, ppm): 132 (C-1), 116( C-2 ), 147 (C-3) , 147 (C-4 ), 116 (C-5 ), 121 (C-6), 72 (C-α ), 36 (C-β ), 56 (4- OCH3 ), 104 (C-1″), 76 (C-2″), 81 (C-3″), 70 (C-4″), 76 (C-5″), 62 (C-6″), 115 (C-α’), 147 (C-β’), 168 (C=O), 127 (C-1′),111 (C-2′), 150 (C-3′), 149 (C-4′), 116 (C-5′), 124 (C-6′), 56 ( 3′- OCH3 ), 102 (C-1″‘), 72 (C-2″‘), 72 (C-3″‘), 73 (C-4″‘), 70 (C-5″‘), 18 (C-6″‘)
Acid Hydrolysis
Compounds isolated (15 mg each) were refluxed, separately, with 0.1 N HCl (30 ml) for 6 hr. Then H2O was added and the mixtures were extracted with CHCl3. The aqueous layer, in each case was neutralized with Ag2CO3 and filtered. The filtrate was evaporated in vacuo and the residue was examined by comparison with authentic sugar samples using silica gel F254 plate, n- BuOH – PrOH – H2O (10:5:4) as developer and anisaldehyde – H2SO4 for detection. All compounds gave glucose ( Rf 0.3 ) and rhamnose ( Rf 0.55 ).
Measurement of DPPH Radical Scavenging Activity
Each EtOH solution (100µl) of compounds 1-5 at various concentrations was added to 1.5×10-5M DPPH/EtOH solution. The reaction mixture was shaken vigorously and the absorbance of remaining DPPH was measured at 530 nm after 30 min. The radical scavenging activity was determined by subtracting the absorbance with that of blank (100%) containing only DPPH and solvent. The samples were prepared using the same dilution procedures and dl –α- tocopherol was used as standard [Abourashed, 2005].
Experimental Animals
Wister rats of either sex weighing 200-250 g were used to determine anti-inflammatory activity. The animals were maintained at 23±20C with a 12-h light and dark cycle, fed a Purina rat chow diet supplied by Grain Silos and Flour Mills Organization, Riyadh, Saudi Arabia, and had free access to food and water.
Determination of Anti-inflammatory Activity
Six rats each were allotted to different treatment groups. Edema was induced in the rats by injecting carrageenin (0.05ml, 1% w/v in normal saline) into the subplantar tissue of the right hind paw [Winter et al.,1962]. Paw volume (mm) was measured with a plethysmometer (7140, Ugo Basile) before carrageenin injection and 0, 1, 2 and 3h thereafter. The edema was reported as the difference between the initial and the final volume. The anti-inflammatory effect was expressed as the percentage inhibition compared with vehicle-treated animals with respect to a reference group treated with phenylbutazone (100 mg/kg). The test compounds (10mg/kg) with distilled water (0.1ml/100g rat) were administered orally 1h before injection of the phlogistic agent.
Results and Discussion
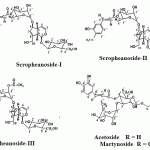
Compound 1, Scropheanoside-I, with formula C31H40O16 through the positive FABMS, with ion beak at m/z 669 [M+H]+ , 691 [M +Na]+ , 323 [C16H18O7] for isoferuloyl-rhamnose fragment and m/z 177 [C10H9O3]+ for isoferuloyl fragment. The IR spectrum of the compound showed absorption bands at 3400 cm-1 (hydroxyl groups), 1700 cm-1 (α,β-unsaturated ester), and 1630 cm-1( conjugated double bond ). 1H-NMR data indicated an olefinic proton at C-3 at, δ 6.3 (1 H, dd) with vicinal coupling J3,4=6.5 with H-4 (δ 5.1 ppm ) and an allylic coupling J3,5 =2 Hz with H-5 ( δ 2.8 ppm ). The presence of H-7 at δ 5.7 ppm and the three homoallylic coupling of H-6 with protons at C-9 and C-10 indicated the presence of carbon, carbon –double bond between C-7 and C-8 of the iridoid nucleus of the compound. The 1H-NMR data showed full agreement with the reported data of aucubin [Abbas and Zayed, 2004, and Pachaly et al., 1994]. The 1H-NMR spectrum exhibited signals for three aromatic protons (δ H 7.09, d, J = 2 Hz, 6.9, d, J = 8 Hz and 7.06, dd , J = 8 &2 Hz) and two olefinic protons ( δ H 6.35, d, and 7.6, d, AB system, JAB =16 Hz ) substantiating the presence of trans isoferuloyl moiety , one methoxy signal (δ H 3.8, s) , one methyl signal (δ H 1.2, d, J = 6.5 Hz ) and two anomeric protons ( δ H 4. 7, d, J = 8 Hz, 4.8, d , J =2 Hz ) indicating the presence of two sugar moieties in the molecule.
 |
Scheme 1
|
On acid hydrolysis, the compound afforded D-glucose and L-rhamnose as sugar moieties. The chemical shifts of protons and carbon atoms were compared with the reported data [Pachaly et al., 1994] which indicated the presence of β-D-glucose and α –L- rhamnose, respectively. The H-1 of the aglycone exhibited long range coupling in the heteronuclear multiple bond connectivity ( HMBC ) spectrum with C-1′ of the glucose unit, H-6 correlated with C-1″ of the rhamnose moiety which indicated that glucose is connected with ether linkage at position 1 and rhamnose with position 6.
The assignments of glucosyl and rhamnosyl protons were also made with the help of 1H-1H –COSY experiments starting with their anomeric protons ( δ H 4.7, d, J = 8 Hz, glucosyl H -1, δ H 4.8, d, J = 2 Hz, rhamnosyl H-1 ) and methylene protons ( δ H 3.6 , dd, J = 12 & 6 Hz, 6′a– H; 3.8 ,dd, J = 12 & 2 Hz, 6′b-H ) in case of glucose and methyl proton (δ H 1.2, d, J = 6.5 Hz, Me-6″ ) in case of rhamnose. The long –rang couplings in the HMBC spectrum also confirmed the above assignments. The 13C-NMR (75 MHz, CD3OD , Table 1) and DEPT spectra of the compound showed 31 carbon atoms for the molecule consisting of two methyls, two methylenes, twenty two methines, four quaternary and one carbonyl carbon atom (in total C31H40 ). The sequential assignments of protons and carbon atoms were made with the help of 1H-1H-COSY and HETCOR spectra. The spectral data of the compound were also compared with those of scropheanoside-I, isolated from Scrophularia koraiensis [Pachaly et al., 1994] which showed a close resemblance. Unequivacally, the structure of the compound was elucidated to be 6-O-α-L-(4-O-isoferuloyl) –rhamnopyranosyl aucubin.
Compound 2, Scropheanoside-II, showed positive FABMS at m/z 685 [M+H]+, 707 [M+Na]+, for molecular formula C31H40O17. The fragment ion peaks at m/z 323 and 177 due to isoferuloyl- rhamnose residue similar to compound 1. Acid hydrolysis of compound 2 gave D-glucose and L-rhamnose. Interpretation of 1H and 13C-NMR indicated an iridoid nucleus with C-9 aglycone of catalpol derivative [ Pachaly et al.,1994]. The 1HNMR showed absorption at δ 6.37 (1 H, dd, J = 6.5 & 2 Hz ) of olefinic H-3 for iridoid nucleus. The resonance of H-7 at δ 3.7 ppm indicated the epoxy- function between C-7 and C-8. The 1H-NMR also showed signals for β –glucose, α- L-rhamnose and isoferuloyl residues similar to compound 1. Analysis of the 13C-NMR spectrum, which showed 31 carbon signals, nine corresponding to aglycone, twelve for sugar moieties and ten for isoferuloyl moiety. Full assignments of the 1H and 13C-NMR signals were accomplished using 1H-1H-COSY and H-C correlation. The absorption at δ c 95 and 84 ppm were assigned to C-1 and C-6, respectively, indicating glycosidation at positions 1 and 6 of the iridoid skeleton.
Table 1: 13C- NMR Spectral Data of Scropheanoside I-III (75 MHz, CD3OD).
| Position | Scropheanoside I | Scropheanoside II | Scropheaoside III |
| 1 | 98 | 95 | 95 |
| 3 | 142 | 142 | 142 |
| 4 | 105 | 103 | 103 |
| 5 | 44 | 37 | 37 |
| 6 | 89 | 84 | 84 |
| 7 | 127 | 59 | 59 |
| 8 | 149 | 67 | 66 |
| 9 | 49 | 43 | 43 |
| 10 | 61 | 61 | 61 |
| 1′ | 100 | 100 | 100 |
| 2′ | 75 | 75 | 75 |
| 3′ | 78 | 78 | 78 |
| 4′ | 71 | 71 | 71 |
| 5′ | 78 | 78 | 78 |
| 6′ | 62 | 62 | 62 |
| 1″ | 101 | 101 | 98 |
| 2″ | 72 | 72 | 71 |
| 3″ | 70 | 70 | 70 |
| 4″ | 75 | 75 | 72 |
| 5″ | 68 | 68 | 68 |
| 6″ | 18 | 18 | 18 |
| 1″‘ | 128 | 128 | 135 |
| 2″‘ | 114 | 114 | 129 |
| 3″‘ | 147 | 148 | 130 |
| 4″‘ | 151 | 151 | 131 |
| 5″‘ | 112 | 112 | 129 |
| 6″‘ | 127 | 123 | 129 |
| α | 116 | 116 | 117 |
| β | 146 | 146 | 147 |
| C=O | 168 | 168 | 167 |
| OCH3 | 56 | 56 | – |
| CO-CH3 | 171 | ||
| CO-CH3 | 172 | ||
| COCH3 | 21 | ||
| COCH3 | 21 |
The 13C-NMR data also showed two olefinic carbons of the iridoid skeleton at C-3 (δ c 142) and C-4 (δ c 103 ). The diglycosidic structure was confirmed by the 13C-NMR spectrum of the compound, where two anomeric carbons at δ c 100 and 101 were observed. All protons of the two sugar units were assigned unambiguously from the shift correlation spectroscopy (COSY) spectrum, and the two sugars were found to be β –glucose and α- L-rhamnose. The significant deshielding of C-4″ of rhamnose (δ c 75) confirmed the placement of isoferloyl residue to C-4″ of rhamnose. 13C-NMR also showed signals corresponding to trans isoferuloyl at δ c 116 (C-α ), 146 (C-β ), 168 (C=O), signals for six aromatic carbons ( δ c 128., 114, 148, 151, 112 & 122) and signals for methoxy function at 56. Finally the compound was proved to be scropheanoside- II by comparing 1H and 13C-NMR spectral data with those of previously reported data [ Pachaly et al., 1994].
Compound 3, Scropheanoside-III, with molecular formula C34H42O17, positive FABMS at m/z 740 [M+NH4]+, m/z 745 [M+Na ]+. The fragment ion peak at m/z 361 [M-C15H12O10] + for acetylcinnamoyl rhamnosyl residue. The 13C-NMR spectrum showed 34 carbon atoms for the molecule containing three methyls, two methylenes,24 methines , two quaternary and three carbonyl carbon atoms (in total C34H42 ). The sequential assignments of protons and carbon atoms were made with the help of 1H-1H (COSY) and HETCOR experiments starting with the easily distinguishable acetal methin proton at δ 5.1 assigned to position 1 (δ c 95) , H-9 ( δ H 2.7, δ c 43) and H-5 ( δ H 2.4 , δ c 37 ).
The 1H- and 13C-NMR spectra exhibited signals for five aromatic protons substantiating the presence of a trans– cinnamoyl moiety, two acetoxy signals (δ H 2.03, s ; 2.15, s ; 2 x Me; δ c 171.5, 171.8, 2 C=O ) and two anomeric protons δ H 4.8, d, J = 8 Hz, δ c 100 ; δ H 5.1, d, J = 2 Hz, δ c 98 ), indicating the presence of two sugar moieties in the molecule. The chemical shifts of proton and carbon atoms were compared with the values reported in the literature [Pachaly et al., 1994, Agarwal, 1992, and Miyase and Mimatsu,1999]. The spectral data of the compound were also compared with those of scropolioside -A and scropolioside-D, previously isolated from Scrophularia scopolii and, Scrophularia ilwensis, respectively [Calis et al.,1988,and 1993] which showed a close resemblance.
Compound 4, Acetoside, showed UV at λmax 320, 280 and 230 nm. The molecular formula C29H36O15 established by positive FABMS at m/z 625 [M+H]+, m/z 642 [M+NH4]+ and m/z 647 [M+Na ]+. 13C-NMR experiments showed three methylene, eighteen methane, one methyl and seven quaternary carbons with two characteristic anomeric carbons at δ c 104 and 102..The 1H-NMR spectrum showed the presence of (E) –caffeic acid and 3,4- dihydroxyphenyl ethanol moieties confirmed by the six aromatic proton signals between δ 6.6—7.0 for 2ABX system, two olefinic protons (AB system, d, JAB = 16 Hz ) at δ 6.3 and 7.6, a benzylic methylene at δ 2.8 (2H, dd, J = 7 &7 Hz) and two non-equivalent protons at δ 3.7 and 4.0 (each 1H, m). Additionally, two doublets of anomeric protons were observed at δ 4.4 (d, J = 8 Hz) and at δ 5.2 (d, J = 2 Hz ) indicating diglycosidic structure. The significant deshielding of C-4″ of the glucose at δ c (70.) confirmed that the caffeoyl residue was attached to C-4″ of glucose . A downfield shift of C-3” of glucose δ c (81) indicated that rhamnose unite was terminal and attached to C-3″ of glucose. The rest of 1H and 13C –NMR data were in full agreement with those reported for acetoside [Pachaly et al., 1994, Li et al., 2000,and Akdemir et al.,1991].
Compound 5, Martynoside, with. molecular formula (C31H40H15) established by positive FABMS m/z 653 [ M+H]+ and m/z 670 [ M+NH4 ]+; The 13C-NMR spectrum of the compound exhibited 31 carbon resonances. The 1HNMR exhibited six aromatic for one ferulic acid and phenylethyl aglycon part;. two olefinic protons (d, each, H- α’ and β’) with JA,B =16 Hz for a trans ferulic acid unit; two methoxy groups; ethylene protons and one secondary methyl group as typically found for rhamnose were assigned. Additionally, two anomeric protons were observed at δ 4.4 (d, J = 8 Hz ) and δ 5.2 (d, J = 2 Hz ) which was consistent with β – glucose unit and α- rhamnose unit, respectively. The 1H-NMR spectrum also confirmed placement of the feruloyl moiety at C-4″ of the glucose moiety (deshielding of H-4″ glucose resonances at δ 4.8, t, J = 9.6 Hz). The signals attributed to the aglycone and an acyl moiety was consistent with the presence of 3- hydroxyl -4 methoxy phenylethanol and ferulic acid, respectively. Because no substituent chemical shift was observed for the rhamnose unit, the deoxy sugar was proven to be terminally linked to the glucose moiety. The complete assignment of most protons and carbons were based on the 1H-1H COSY, HMQC and HMBC experiments. The structure of the compound was confirmed to be martynoside by comparison with published data [ Pachaly et al., 1994].
Biological Activity
The isolated glycosides were also screened for their anti-oxidative and anti-inflammatory activities
Anti-oxidant Activity [Molgard and Ravn, 1988, Jimenez and Riguera , 1994, Xiang et al., 1996,and Gao et al., 2000]
Martynoside and acetoside have potent radical scavenging activity which is more than dl– α- -tocopherol as natural antioxidant. Their IC50 values are as follows: Martynoside 3.6×10 -4 M; acetoside 2.4×10 -4 M and dl-α- tocopherol 4.8×10 -4 M . Inhibitory activity of acetoside was higher than that of martynoside .
Table 2: Effect of Compounds 1-5 on Right Rat Paw Swelling Induced By Carrageenin.
| Group (n =6 ) | Dose | Carrageenin-induced edema
|
|
| Mean increase in paw Inhibition (%)
Volume after 3 h |
% of inhibition | ||
| Control (only carrageenin) | 0.05 ml (1% w/v) | 50 ± 0.05 | — |
| Compound 1 | 10 mg/kg | 35 ± 0.02 | 26 |
| Compound 2 | 10 mg/kg | 37 ± 0.03 | 22 |
| Compound 3 | 10 mg/kg | 33 ± 0.03 | 31 |
| Compound 4 | 10 mg/kg | — | — |
| Compound 5 | 10 mg/kg | — | — |
| Phenylbutazone (standard) | 100 mg/kg | 20 ± 0.01 | 60 |
Values are expressed as ± S.E.M.
Anti-inflammatory Activity
The results are presented in Table 2.The phenylpropanoid glycosides decreased edema in the range of 22-31 % at a dose of 10 mg/kg after 3 h with respect to the control group treated only with carrageenin, against phenylbutazone (60 % decrease) at a dose of 100 mg/kg, which indicated that the test compounds had moderate anti-inflammatoryactivity. Scropheanoside- III (31% decrease) and Scropheanoside- I (26 % decrease). It may be concluded that compound containing a cinnamoyl moiety as found in Scropheanoside- III has significant activity.
Acknowledgements
The author is deeply thankful to Dr. Marie- Luise Koch, Institute of Pharmaceutical Biology, Bonn University, Germany, for carrying the spectroscopic measurement. The author would also like to thank Dr. Kamal EL Tahir, Professor of Pharmacology, College of Pharmacy, KSU, Saudi Arabia for the pharmacological studies.
References
- Abbas, F. and Zayed, R. Iridoid glycosides from Scrophularia xanthoglossa Bioss. Family Scrophulariaceae. Egypt J. Pharm. Sci. 45: 53-65(2004).
- Abourashed, EA. Thin-layer densitometry as an alternative tool in the quantitative evaluation of the free radical scavenging activity of natural antioxidants. Z. Naturforsch 60 b : 1-7(2005).
- Agarwal, PK. NMR spectroscopy elucidation of oligosaccharides and glycosides. Phytochemistry 31: 3307-3330 (1992).
- Akdemir, Z. , Calis, I. and Junior, P. Iridoid and phenylpropanoid glycosides from Pedicularis Phytochemistry 30: 2401(1991).
- Calis, I. , Gross, , Winkler, T. and Sticher, O. Isolation and structure elucidation of two acylated iridoid glycosides from Scrophularia scopolii. Planta Med. 54: 168 -170 (1988).
- Calis, I. , Zor, M. , Basaran, AA. , Wright, AD. and Sticher, O. Karsoside and Scropolioside D, two new iridoid glycosides from Scrophularia ilwensis. Nat. Prod. 56: 606-609(1993).
- Gao, J. , Igarashi, K. and Nukina, M. Three new phenylethanoid glycosides from Caryopteris incana and their antioxidative activity. Pharm. Bull. 48: 1075 – 1078 (2000).
- Jimenez, C. and Riguera, R. Phenylethanoid glycosides in plants: structure and biological activity. Prod. Rep. 11: 591- 606 (1994).
- Li, YM. , Jiang, SH. , Gao, WY. and Zhu, DY. Phenylpropanoid glycosides from Scrophularia Phytochemistry 54: 923-925 (2000).
- Miagahid, AM. Flora of Saudi Arabia, University Libraries, King Saud University, Riyadh, Saudi Arabia, 3rd. ed. , p.507(1989).
- Miyase, T. and Mimatsu, A. Acylated iridoid and phenylethanoid glycosides from the aerial parts of Scrophularia nodosa. Nat. Prod. 62:1079 – 1084 (1999).
- Molgard, P. and Ravn, H. Evolutionary aspects of caffeoyl ester distribution in dicotyledons. Phytochemistry 27: 2411-2421(1988).
- Pachaly, P. , Barion, J. and Sin, KS. Isolierung und strukturaufklarung neuer iridoidglycoside aus Scrophularia koraiensis. Pharmazie 49:150 – 155(1994).
- Xiang, Q. , Kadota, S. , Tani, T. and Namba, T. Antioxidative effects of phenylethanoids from Cistanche deserticola . Pharm. Bull. 19: 1580 – 1585(1996).
- Winter, C. , Risely, E. and Nuss, GW. Carageenin- induced edema in paw of rat as an assay of anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. III: 544 -547(1962).

This work is licensed under a Creative Commons Attribution 4.0 International License.





