How to Cite | Publication History | PlumX Article Matrix
C. Uma* and K. Chandra kumar
Department of Biochemistry, School of Life sciences, Karpagam University, Coimbatore - 641 021 India.
Corresponding Author E-mail:umaradhakrishnan29@gmail.com
ABSTRACT: Invertase was isolated from selected soil micro organism namely A.fumigatus and P. brevicompactum, the enzyme was purified by ammonium sulfate precipitation and Ion Exchange chromatography. The purification fold was 7.85 for A.fumigatus and 6.35 for P.brevicompactum. The enzyme showed maximum activity at pH 6 and optimum temperature was 50o C at a substrate concentration of 1.0 gm. The activiy of the enzyme was inhibited by divalent metal ion Zn 2+ where as activated by Na+. Immobilisation by sodium alginate method increased the stability of the enzyme.
KEYWORDS: Invertase; Aspergillus fumigatus; Penicillium brevicompactum.
Download this article as:| Copy the following to cite this article: Uma C, Kumar K. C. Purification And Characterization Of Invertase From Aspergillus Fumigatus And Penicillium Brevicompactum. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Uma C, Kumar K. C. Purification And Characterization Of Invertase From Aspergillus Fumigatus And Penicillium Brevicompactum. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9599 |
Introduction
Invertases or β d- fructofuranosidase (EC No. 3.2.1.26) are special kind of enzymes that catalyze the hydrolyses of sucrose. The enzyme cleaves α-1-4 glycosidic linkage between α-D-glucose and β-d-fructose molecules of sucrose by hydrolyse producing monosaccharides such as glucose and fructose. β d- fructofuranosidase are extracellular as well as intracellular enzymes (Mahmoud, 2007). Invertase also hydrolyses bête fructans such as raffinose into simple sugars (Baig et al 2003). In contrary to most other enzymes, invertase exhibits relatively high activity over a broad range of pH (3.5 – 5.5), with optimum near 4.5. The enzyme activity reaches a maximum at about 55 C (Fontana et al. 1992).
Invert syrup production by microbial invertase is not widespread because of ease in chemical hydrolysis and high price of the enzyme. It is also used in the production of calf feed preparation, assimilation of alcohol in fortified wines and in manufactural inverted sugars as food for honeybees. (Chou and Jasovsky, 1993)
In this present study we report the purification and biochemical characterization of thermo stable extracellular invertase produced by filamentous fungi A.fumigatus and P.brevicompactum by using sugarcane bagasse as substrate. We have studied in detail the effects of various conditions affecting the extracellular invertase activity.
Materials and methods
Isolation and Enumeration of Fungi From Soil
A large number of fungi of different groups are found in soil. They constitute the major place among soil microorganisms .A small amount of soil sample was collected from four corners and centre of the sugarcane field at pollachi by making a ‘V’ shaped pit.They were mixed to make one lot. 10g of the soil sample was weighed and then the sample was serially diluted with sterile distilled water. 1.0 ml of the diluted soil suspension was transferred aseptically into the PDA Agar plates (Supplemented with aureomycin antibiotic each 30 mg /L). Gently rotate the plate so as to spread the suspensions on the medium. The plates were incubated at 300 C for 4-5 days
Fermentation technique
Production of β–fructofuranosidase was carried out by shake flake technique using 250 ml Erlenmeyer flasks. Fifty ml of fermentation medium was transferred to each Erlenmeyer flasks. The cotton-plugged flasks were autoclaved at 15 lbs/inch2 pressure for 15 minutes and cooled in room temperature. It was then inoculated with 24 hours old culture of A.fumigatus and P.brevicompactum and incubated at the optimum temperature 300C. At the 4th day of incubation the biomass, invertase activity and protein content was estimated. Flasks were then incubated in an orbitol shaker at 30º C temperature. (Montiel-Gonzalez et al. 2002)
Purification of Invertase
All steps of enzyme purification were carried out at 4oC. The crude enzyme was purified by the method proposed by Dahot et al. 1996 in a stepwise process.
Step I- Ammonium Sulphate precipitation
Solid Ammonium Sulphate was slowly added to crude extract with gentle stirring and the proteins that precipitsted at 30 % saturation was collected by centrifugation for 30 minutes at 15,000rpm. The precipitate was dissolved in 10 mM Tris Hcl buffer and dialysed against distilled water over night.
Step II- Ion Exchange Chromatography
10 ml of pooled enzyme was loaded on to a DEAE cellulose chromatographic column (1cm X 10cm) equilibrated with tris Hcl buffer, 100 mM, pH 7.5. The enzyme was eluted with a linear salt concentration gradient (NaCl 250-1500mM) in the same buffer.10 ml of fractions were collected at the flow rate range of 20ml/hr.
Characterization of Invertase Activity
The characterization of commercial processes but also for the meaningful determined by measuring the enzyme activity by varying the single parameter such as pH, temperature and substrate concentration of the medium keeping the remaining parameter unaltered. The effect of metal ions and immobilization on invertase activity is also determined. (Arruda et al. 1999; Meena and Raja, 2003)
Protein Estimation
Quantitative estimation of the protein content of individual fraction obtained after different steps was done by the method of Lowry et al. 1956
Enzyme Assay
b-Fructofuranosidase assay was determined by measuring the reducing sugars released by the hydrolysis of sucrose. The reaction was carried out at 300C for 5 minutes. The reducing sugars released in the reaction mixture were assayed by DNS method. The cell free extract obtained after centrifugation is used as the enzyme source for determining the crude enzyme activity. (Al-Bakir et al. 2007)
Assay Mixture
The determination invertase activity was carried out of at 300C in a mixture of 1.0ml of 0.02M acetate buffer (pH 6), 1.0 ml of 0.03 M sucrose solution and 0.1 ml of cell free extract.
The mixture was incubated at room temperature (-300C) for 5 minutes after making up the volume of the mixture to 4.0 ml with distilled water .The hydrolysis was stopped by adding 2.0 ml of DNSA reagents and then invertase activity was assayed as described using glucose or fructose as a standard. (Al-Bakir et al. 2007)
Enzyme Units
One unit of invertase (IU) was defined as the amount of enzyme which liberated / mg of product / minute /ml under the assay condition. (Cairns et al. 2006)
Results and Discussion
The crude invertase enzyme from the Aspergillus fumigatus and Penicillium brevicompactum was purified by ammonium sulphate precipitation, dialysis and DEAE cellulose chromatography. The purified enzyme had low protein content when compared to the crude enzyme. The specific activity of the enzyme was increased after purification.
Invertase from A.fumigatus and P.brevicompactum was successively purified by DEAE cellulose column chromatography. Table 1 and 2 shows the elution profile of ion-exchange chromatography of crude Aspergillus fumigatus and Penicillium brevicompactum extracts on CEAE cellulose column.
The crude extract of the enzyme from Aspergillus fumigatus had 9970 enzyme units with a specific activity of 27.0 units mg-1. The crude extract of the enzyme from Penicillium brevicompactum had 9940 enzyme units with a specific activity of 31.0 units mg-1.
The crude extract of Aspergillus fumigatus and Penicillium brevicompactum was subjected to 30% ammonium sulphate precipitation. The precipitates had 7012 units and 6360 respectivily. The specific activity of Aspergillus fumigatus and Penicillium brevicompactum was 44.50 units mg-1 protein with 1.65 fold increases and 47.25 units mg-1 protein with 1.52 fold increases.
Similar observation was obtained by Santiago et al. 1967, the enzyme purified by using column chromatography produced from Saccharomyces strain. This was also supported by Shaheen I and Haq Nawaz Bhatti (2007), the enzyme purified by using column chromatography produced from newly isolated Fusarium spp., under solid state fermentation.
The purified enzyme of Aspergillus fumigatus & Penicillium brevicompactum incubated with acetate buffer with varying pH 3.0 to 8.0. The enzymes show maximum activity at pH 6. (Figure 1) This data obtained was supported by Hoi-Seon-Lee et al. 1996, the purified invertase activtity reaches maximum at pH 6.5 produced from carrot. This was also supported by Kiel et al. 1977, the optimum pH for the invertase activity is 6.0 produced from Actinomyces viscosus.
Figure 2 shows the effect of temperature on the enzyme activity was studied by incubating the enzyme mixtures obtained from Aspergillus fumigatus and Penicillium brevicompactum at varying temperatures within the range of 200C to 800C. The optimum temperatures for the enzyme activity for both organisms were found to be at the temperature of 500C.
This result obtained was also supported by Kuramitsu, 1973, the invertase activity reaches maximum at the temperature of 470C produced from cariogenic streptococcus mutants. This was also supported by Weerasooriya et al. 2003, the optimum temperature for the invertase activity was 370C obtained from the flowers of Madhuca longifolia.
The effect of substrate concentration on the enzyme activity was studied by incubating the purified enzymes from Aspergillus fumigatus and Penicillium brevicompactum at varying concentrations of sucrose ranging from 0.5% to 2.5% under standard assay conditions. The invertase from Aspergillus fumigatus enzyme activity reaches maximum at the substrate concentration of 1g/100ml and invertase activity of Penicillium brevicompactum enzyme reaches maximum at the substrate concentration of 1.5g/100ml (Figure 3).
The activity of the enzyme produced by the Cladosporium sp., was subjected to alteration with varying substrate concentration. The result revealed that 1.0 ml of enzyme can hydrolyse a maximum of 0.4gm of substrate. (Gogoi et al. 1998.)
The purified enzymes of Aspergillus fumigatus & Penicillium brevicompactum were incubated with different cations. Invertase activity was stimulated by Cacl2 and NaCl. The elevated concentrations of Mg2+, Zn2+ and Cu2+ drastically inhibited the invertase activity. Zn2+ was found to be the potent inhibitor of invertase (Figure 4).
This was also supported by Kestwal et al. 2008, the invertase activity was stimulated by low concentrations of Mncl2 and NaCl from A.ochraceus. invertase from R.gultinis and F.solani were also activated by Na+ and Mg2+ . Thia was also supported by FernandoPrado et al. 1985, the elevated concentration of Ba2+, Cu2+, NH4+ and Zn2+ inhibits the invertase activity from Ricinus communis.
Immbilization enhances the invertase activity from Aspergillus fumigatus and Penicillium brevicompactum. The use of free enzyme in industrial application has been limited, mainly due o the high cost of enzymes, their instability and irrecoverability. This can be overcome by immobilization. The invertase activity before and after immobolization is given in table 3.
Table 1: Purification of invertase from Aspergillus fumigatus.
|
Description |
Invertase Activity (Units) | Total protein (mg\ml) | Specific Activity
Units/mg |
Purification fold |
|
Crude extract |
9970 |
369.45 |
27 |
1 |
|
30% Ammonium sulphate saturation |
7012.50 |
157.58 |
44.50 |
1.65 |
|
DEAE Cellulose Column Chromatography |
424.25 |
2.01 |
212 |
7.85 |
Table 2: Purification of invertase from Penicillium brevicompactum.
| Description | Invertase Activity (Units) | Total protein
(mg\ml) |
Specific Activity
(Units/mg) |
Purification fold |
|
Crude extract |
9940 |
320.64 |
31 |
1 |
|
30% Ammonium sulphate saturation |
6360 |
134.60 |
47.25 |
1.52 |
| DEAE Cellulose Column Chromatography |
393.75 |
1.99 |
197.50 |
6.35 |
Table 3: Effect of immobilization on invertase.
|
Enzyme Source |
Enzyme Sample
|
Invertase Activity (IU\ml) |
|
Aspergillus fumigatus |
Crude extract |
15.21 |
|
Immobilized |
23.49 |
|
|
Penicillium purpogenum |
Crude extract |
14.95 |
|
Immobilized |
21.65 |
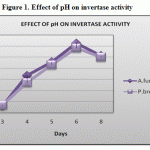 |
Figure 1: Effect of pH on invertase activity.
|
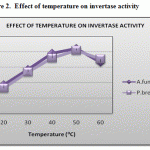 |
Figure 2: Effect of temperature on invertase activity.
|
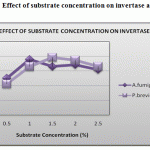 |
Figure 3: Effect of substrate concentration on invertase activity.
Click here to View figure |
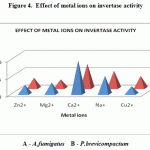 |
Figure 4: Effect of metal ions on invertase activity.
|
Conclusion
From the present study, we could see that parameters like pH, temperature, substrate concentration, metal ions and immobilization had different effect in the enzyme characterization after purification. Hence purified form of the enzyme Invertase from the two fungal strains can be used in food industries.
References
- Al-Bakir and Whitaker JR 2007 Purification and characterization of invertase from dates. (Phoenix dactylifera). J. Food. Biochem. 2 133-160
- Arruda LMO and Vitolo M. 1999 Characterization of invertase entrapped into calcium alginate beads. J. Appl. Biochem. Biotech. 81 23-33
- Baig MA, Shafiq K, Sikander Ali and Ul-Haq I. (2003) Effect of nitrogen and phosphorus on the biosynthesis of β-fructofuranosidase. Pak. J. Biosci. 6 591-595
- Cairns AJ, Howarth CJ, Pollock C 2006 Characterization of acid invertase from the snow mould Monographella nivalis: A mesophilic enzyme from a Psychrophilic fungus. J. New Physiol. 130 391-400.
- Chou CC, Jasovsky GA 1993 Advantages of Escorb T M precoats in liquid sugar production . Inter sugar J. 95 425-430.
- Dahot MU and Hanif NM 1996 Purification and some properties of invertase from Anchras sapota fruit. J. Islamic Academy of Science. 9 31-36.
- Doaa AR Mahmoud 2007 Immobilization of invertase by a new economical method using wood sawdust waste. J. Basic Appl. Sci. 4 364-372.
- Fernando E, Prado, Marta AV, Olga LF, Antonio RS 1985 Purification and characterization of Ricinus communis invertase. J. Biol. Chem. 260 4952-4957.
- Fontana A, Ghommidh C, Guiraud JP, Narroca JM 1992 Continuous alcoholic fermentation of sucrose using flocculating yeast. The limits of invertase activity. J. Biotech. Letters. 14 505-510.
- Gogoi BK, Pillai KR, Nigam JN, Bezbaruah RL 1998 Extracelluar α Amylase and invertase from amylolytic yeast Saccharomycopsis fibuligera .Indian Journal of Microbiology. 38 15-19.
- Gonzalez AM, Fernandez FJ, Viniegra GG, Loera O 2002 Invertase production on solid state fermentation by A.niger improved by parasexual recombination. J. Appl. Biochem. Biotech. 102 63-70.
- Kestwal BD, Karve MS, Kakada B, Pillai VK 2008 Invertase inhibition based electrochemical sensor for the detection of heavy metal ions in aqueous system. J. Bioosens. Bioelect 1770 1506-1512.
- Kiel RA, Tanzer JM, Woodiel FN 1977 Identification, separation and preliminary characterization of invertase and β-galactosidase in Actinomyces viscosus. J. Infect. Immunol. 16 81-87
- Kuramitsu HK. 1973 Characterization of invertase activity from cariogenic Streptococcus mutants. J. Bacteriology. 115 1003-1010
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951 193 265–275.
- Santiago G, Oliver L 1967 Purification of internal invertase of yeast. J. Biol. Chem. 243 1567-1572.
- Shaheen I and Haq Nawaz Bhatti T.A, 2007 Production, purification and thermal characterization of invertase from newly isolated Fusarium spp, under solid state fermentation. J. Food Sci. Tech. 43 1152-1158.

This work is licensed under a Creative Commons Attribution 4.0 International License.





