How to Cite | Publication History | PlumX Article Matrix
Radical Scavenging Activity of Clitoria Ternatea Leaf Extracts
A . Jayachitra1 and P. R. Padma2
1Department of Biochemistry and Bio-technology, Sourashtra College, Madurai - 625 004 India. 2Reader,Department of Biochemistry, Biotechnology and Bioinformatics, Avinashilingam University, Coimbatore India. Correspnding Author E-mail: jchitra21@gmail.com
ABSTRACT: Free radical scavenging assays were performed in both blue flowered leaf and white flowered leaf of Clitorea ternatea and analyse their ability to scavenge the oxidants like DPPH, SO·, NO, and OH· by using different solvents like petroleum ether, butanol, chloroform, ethanol and water. The ethanolic extracts offered the maximum scavenging in all the assays . The effects of white flowered leaves were more efficient than those of blue flowered leaves.
KEYWORDS: Clitoria ternatea; antioxidants; free radical; different solvents.
Download this article as:| Copy the following to cite this article: Jayachitra A, Padma P. R. Radical Scavenging Activity of Clitoria Ternatea Leaf Extracts. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Jayachitra A, Padma P. R. Radical Scavenging Activity of Clitoria Ternatea Leaf Extracts. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9518 |
Introduction
Antioxidants act as a defense mechanism that protects against oxidative damage, and include compounds and repair enzymes to remove or repair damaged molecules. However, the natural antioxidant compounds become important (Malpure et al., 2006). Antioxidants can prevent/retard the oxidation caused by free radicals and sufficient intake of antioxidants is supposed to protect against diseases (Celiktar et al., 2007). Nitric oxide radical is a labile compound, which has a brief half life and is rapidly converted to the stable end products nitrite (NO2¯) and nitrate (NO3¯) (Everekiloglu et al., 2003). One of the plants that deserves attention is Clitoria ternatea (Sanskrit-Sankupushpam) belongs to the family Fabaceae, is widely used in traditional Indian system of medicine as a brain tonic (Gomez and Kalamani, 2003).Clitoria ternatea is a perennial twinning herb bearing blue or white flowers .The present study is the new one concentrating on the antioxidant activity and to analyze the free radical scavenging, biomolecular protecting and antioxidant modulating effects .
Materials and Methods
Materials
TLC plates (Silica gel 60 F254, Merck), DPPH (0.4mM in methanol), DPPH (0.3 mM in methanol), Methanol,EDTA (0.1M containing 1.5 mg NaCN),Nitroblue tetrazolium (NBT, 1.5mM),Riboflavin (0.12mM),Phosphate buffer (0.06 M, pH 7.6),Dimethyl sulphoxide (DMSO),Phosphate buffered saline (PBS), pH 7.2,Sodium nitroprusside (100mM),Griess reagent (1% sulphanilamide, 2% phosphoric acid and 0.1% napthylethylene diamine dihydrochloride),Ferric chloride (0.1 mM),EDTA (0.1mM),H2O2 (1mM),Ascorbate (0.1mM),KH2PO4-KOH buffer (20mM, pH 7.4,Deoxyribose (2.8mM),Thiobarbituric acid (TBA, 1%),All other reagents were of the highest grade available commercially.
Solvent Extraction
As the initial step in the second phase, the leaves were serially extracted into cold solvents of increasing polarity. The solvents used were petroleum ether, benzene, chloroform, ethanol, methanol and water. The residues of the different solvent extracts were weighed and redissolved in a known volume of the same solvent in which they were extracted.
Free Radical and Oxidant Scavenging Effects of the Leaves of
Clitoria Ternatea
The leaf extracts were tested for their scavenging activity against the stable free radical DPPH (2,2’-diphenyl-1-picryl hydrazyl). The ability of the leaf extracts to scavenge DPPH was studied in a dot plot rapid screening assay and quantified using a spectrophotometric assay as proposed by Soler-Rivas et al. (2000) and Mensor et al. (2001) respectively.
Rapid Screening of Antioxidant Activity by Dot Plot Assay
Antioxidants react with diphenyl-1-picryl hydrazyl (DPPH) and convert it to diphenyl-picryl hydrazine. The degree of discolouration from purple to yellow colour can be used as a measure of the scavenging potential of antioxidant extract, Aliquots (3µl) of Clitoria ternatea extracts were spotted on a TLC plate and allowed to dry. The TLC plate bearing the dry spots was placed upside down for 10 seconds in the solution of DPPH. The spots exhibiting radical scavenging, antioxidant activity showed up as yellow spots in a violet background. The intensity of the yellow colour depends on the amount and nature of the radical scavenger present in the spot.
Dpph Photometric Assay
The ability of the leaf extracts to bleach DPPH can be quantified using a spectrophotometric assay, the extent of scavenging causing a proportionate change in the absorption at 518nm. An exact amount (0.5ml) of the methanolic solution of DPPH was added with 20 µl of the leaf extracts in the different solvents and the crude aqueous extract (corresponding to 4mg) and 0.48ml of methanol, and allowed to stand at room temperature for 30 minutes. Methanol served as the blank. After 30 minutes, the absorbance was measured at 518nm and converted into percentage radical scavenging activity as follows:
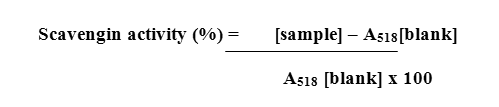
Inhibition of in Vitro Generation of Superoxide and Nitric Oxide
In the present study, the efficiency of the leaf extracts of Clitoria ternatea in inhibiting the in vitro generation of superoxide and nitric oxide were studied using the methods elaborated by Winterbourn
et al. (1975) and Green et al. (1982) respectively.
Determination of Superoxide Generation in Vitro
The extent of superoxide generation was studied on the basis of inhibition of production of nitroblue tetrazolium formazone by the plant extracts measured spectrophotometrically at 560nm.The assay tubes contained 0.02ml of the plant sample (corresponding to 20mg extract) with 0.2ml EDTA, 0.1 ml nitroblue tetrazolium, 0.05ml riboflavin and 2.64ml phosphate buffer. The control tubes were set up without the leaf extracts, where DMSO was added. The initial optical densities of the solutions were recorded at 560nm and the tubes were illuminated uniformly with the fluorescent lamp for 30 minutes. A560 was measured again and the difference in O.D was taken as the quantum of superoxide production. The percentage inhibition by the leaf samples was calculated by comparing with the O.D of the control tubes.
Determination of Nitric Oxide Generation in Vitro
Aqueous solution of sodium nitroprusside spontaneously generates nitric oxide (NO) at physiological pH, which interacts with oxygen to produce nitrite ions, which is measured spectrophotometrically at 546nm.The reaction mixture containing 0.3ml of sodium nitroprusside, 2.68ml PBS and 20µl of leaf extract (20mg) was incubated at 25˚C for 15 minutes. Control tubes (100% generation) were prepared without the leaf extracts. After incubation, 0.5ml of the Griess reagent was added. The absorbance of the chromophore formed, indicative of the quantum of NO generated, was read at 546 nm.
Determination of Hydroxyl Radical Scavenging Activity
The hydroxyl radical scavenging activity of the leaf extracts was quantified by the method reported by Elizabeth and Rao (1990).The damage to deoxyribose was quantified as thiobarbituric acid reactive substances, which is also taken as a measure of hydroxyl radical scavenging activity of the leaf extracts. The reaction mixture (0.1ml) contained 0.1ml of deoxyribose, 0.1ml of FeCl3, 0.1ml of EDTA, 0.1ml of H2O2, 0.1ml of ascorbate, 0.1ml of KH2PO4-KOH buffer and 20µl of leaf extracts (20mg) in a final volume of 1.0ml. The reaction mixture was incubated for 1 hour at 37˚C. Then 1.0ml of TBA was added and heated in a boiling water bath for 20 minutes. The pink colour produced was measured at 535 nm in a spectrophotometer. Deoxyribose degradation was measured as TBARS and the per cent inhibition was calculated.
Statistical Analysis
All the parameters studied were subjected to statistical treatment using SigmaStat Statistical package (version 3.1). one way ANOVA, followed by post-hoc analysis using Fisher’s LSD was adopted to all the parameters under study to test the levels of statistical significance.
Results
Free Radical and Oxidant Scavenging Effects of the Leaves of Clitoria Ternatea- Dot Plot Assay
In an effort to understand the nature of the active principles in the leaves, they were serially extracted into solvents of increasing polarity (petroleum ether, benzene, chloroform, ethanol and methanol).
The leaf extracts were tested for their scavenging activity against the stable free radical DPPH by a qualitative rapid dot plot assay and quantified spectrophotometrically.
The picture obtained in the dot plot assay is shown in Plate 1.In this assay, the maximum DPPH scavenging activity was expressed by the ethanolic extract, followed by the methanolic extract. Considerable scavenging effects were also noted with the other extracts
Dpph Dot Plotassay
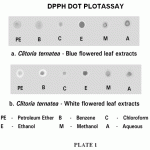 |
Plate 1
|
Photometric Assay
The radical scavenging effects of Clitoria ternatea leaves were also quantified using the photometric assay. To confirm the observations in dot plot rapid screen, the photometric quantification of the extent of DPPH scavenging by different solvent extracts was followed. The results, expressed as per cent scavenging, are represented in Figure 1.
The results revealed that the maximum extent of DPPH scavenging activity was elicited by the ethanol extract, followed closely by methanol extract and then by the aqueous extract. This trend was observed in both the leaves. The white flowered leaves exhibited more DPPH scavenging activity than the blue flowered ones.
Following this, the extracts were individually tested for their effects on the in vitro generation of SO and NO. Figures 2 and 3 shows the per cent inhibition of superoxide and nitric oxide radical generation in vitro by the leaves of Clitoria ternatea and Figure 4 shows the hydroxyl radical scavenging activity.
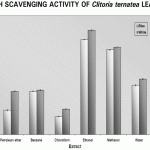 |
Figure 1
|
 |
Figure 2: Clitoria Ternatea Leaves On Superoxide Generation In Vitro
|
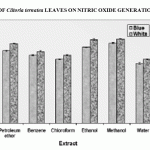 |
Figure 3: Effect Of Clitoria Ternatea Leaves On Nitric Oxide Generation In Vitro
|
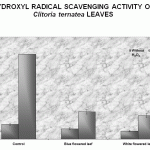 |
Figure 4: Hydroxyl Radical Scavenging Activity of Clitoria Ternatea Leaves
|
Both the varieties of Clitoria ternatea leaf extracts exhibited considerable scavenging of both SO and NO generated in vitro. The highest inhibitory effect was observed in the ethanolic extract of the leaves, with the white flowered ones faring better over the blue flowered ones.
The extracts of Clitoria ternatea leaves effectively scavenged hydroxyl radicals. It can be observed from the results that white flowered leaves rendered better protection than the blue flowered leaves.
Discussion
Clitoria ternatea leaves, extracted serially into solvents of increasing polarity (petroleum ether, benzene, chloroform, ethanol, methanol and water) were tested for their free radical scavenging activity against DPPH, superoxide (SO●), nitric oxide (NO) and hydroxyl radicals (OH●).
The DPPH scavenging ability of medicinal plants has been attributed to several components, including phenolics (Beppu et al., 2003; Valentao et al., 2003; Bartolome et al., 2004), flavonoids (Shetgiri and D’Mello, 2003) and meroterpenoids (Fisch et al., 2003). The DPPH scavenging activity of selected herbal medicinal plants was found to be related to their antimutagenic activity (Gulcin et al., 2004). Many medicinal plants have been analyzed and reported for their DPPH scavenging activity. Jainu and Shyamaladevi (2005) have shown that a methanolic extract of Cissus guandiangularis exhibited significant DPPH scavenging effect. In the present study, the maximum extent of DPPH scavenging activity was elicited by the ethanol extract followed by the methanol extract and then by the aqueous extract. The white flowered leaves exhibited more DPPH scavenging activity than the blue flowered ones.
Clitoria ternatea leaves were also tested for their scavenging activity against superoxide radical generation. Many reports in the literature associate the SO●scavenging activity of plants and their components with strong antioxidant activity. The white flowered leaves exhibited a better radical scavenging activity in ethanol extract than the blue flowered leaves. Superoxides are believed to underlie many of the oxidative changes. Superoxides react with nitric oxide forming peroxynitrite (ONOO–), which rapidly causes oxidative event which rapidly causes oxidative events including LPO, DNA damage and cell death s (Cowell and Rusell, 2004). Excessive production or inadequate removal of O2–l has been implicated in the pathogenesis of many cardiovascular and other diseases including atherosclerosis, hypertension and diabetes (Fukai et al., 2000). Superoxide has been reported to directly initiate lipid peroxidation (Yen and Duh, 1994), if the dismuted superoxide derivative is not immediately scavenged by antiperoxidative enzymes. The present study shows that the SO● scavenging action is higher in polar solvent extracts of Clitoria ternatea leaves than the non-polar extracts. However, the non-polar solvent extracts also exhibited considerable SO● radical inhibition.
Another radical that was studied in the present investigation was nitric oxide (NO). NO, which is the primary RNS, is a key signaling molecule in physiological and pathological conditions .Quercetin, a polyphenolic flavonoid compound reacts strongly with hydrogen peroxide and effectively scavenges NO (Geetha et al., 2005) Similarly, several specific components of plants have been shown to possess potent NO inhibitory activities (Banskota et al., 2003). Nitrogen is taken into the body as nitrates, nitrites, peptides, proteins and amino acids. The metabolites of nitrogen include nitric oxide, higher oxides of nitrogen and peroxy nitrite. Although NO is a free radical, it is probably insufficiently reactive to attack DNA directly. By contrast, N2O3, HNO2 and ONOO– can nitrate and deaminate DNA and cause strand breakage and mutations (Ishii et al., 2001).
In the present study, both the varieties of Clitoria ternatea exhibited considerable scavenging of both SO and NO generated in vitro. The highest inhibitory effect was observed in the ethanolic extract of leaves, with the white flowered ones faring better over the blue flowered ones. The OHl radical scavenging activity caused the maximum damage to deoxyribose. This damage was very effectively counteracted by the leaf extracts of Clitoria ternatea. The white flowered leaves rendered better protection than the blue flowered leaves.
Based on the above results, in order to reap the maximum benefits of the Clitoria ternatea leaf extracts, an ethanolic extract of the leaves was prepared and used in the subsequent studies. In all the contents the white flowered leaves were higher than the blue flowered leaves.
Acknowledgments
This work was supported by Avinashilingam university,Coimbatore-43
References
- Bartolome, B., Nunez, V., Monagas, M. and Cordoves, C.G. (2004), In vitro antioxidant activity of red grape skins, Curr. Food Res. Technol., 218, 173-177.
- 2., Beppu, H., Koike, T., Shimpo, K., Chihara, T., Hoshino, M., Ida, C. and Kuzuya, H. (2003), Radical scavenging effects of Aloe arboresces miller on prevention of pancreatic islet b-cell destruction in rats, J. Ethnopharmacol., 89, 37-45.
- Banskota, A.H., Tezuka, Y., Adnyana, I.R., Xiong, Q., Hase, K., Tran, K.Q., Tanaka, K., Saiki, J. and Kadota, S. (2000), Hepatoprotective effect of Commbretum quadrangulare and its constituents, Biol. Pharm. Bull., 23, 456-460.
- Celiktar, O.Y., Girgin, G., Orhan, H., Nichers, H.J., Bedir, E. and Sukan, F.V. (2007), Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus officinalis extracts with focus on location and harvesting times, Eur. Food Res. Technol., 24, 443-451.
- Cowell, R., M. and Rusell, J.W. (2004), Nitrosative injury and antioxidant therapy in the management of diabetic neuropathy, J. Investig. Med., 52, 33-34.
- Evereklioglu, C., Er, H., Doganay, S., Ceknien, M., Turkoz, Y., Ottu, B. and Ozerol, E. (2003), Nitric oxide and LPO are increased and associated with decreased antioxidant enzyme activities in patients with age related mascular degeneration, Documenta Ophthalmologica, 106, 129-136.
- Elizabeth, K. and Rao, H.N.A. (1990), Oxygen radical scavenging activity of curcumin, Int. J. Pharm., 58, 237-240.
- Fisch, K.M., Bohm, V., Wright, A.D. and Konig, G.M. (2003), Antioxidative meroterpenoids from the brown algae- Cystoseria crinite, J. Nat. Prod., 66, 968-975.
- Fukai, T., Folz, R.J., Landmesser, U. and Harrison, P.G. (2000), Extracellular superoxide dismutase and cardiovascular disease, J. Card., 5, 239-249.
- Gomez, S.M. and Kalamani, A. (2003), Butterfly pea (Clitoria ternatea): A nutritive multipurpose forage legume for the tropics–An Overview, Pak. J. Nutr., 2, 374-379.
- Green, L.C., Wagner, P.A., Glogowski, J., Skipper, P.L., Wishnok, J.S. and Tannenbaum, S. R. (1982), Analysis of nitrate, nitrite and (15N) Nitrite in biological fluids, Anal., Biochem. 126, 131-138.
- Ishii, N., Patel, K.P., Lane, P.H., Taylor, T., Bain, K., Murad, F., Pollock, J.S. and Carmines, P.K. (2001), Nitricoxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus, J. Am. Soc. Nephrol., 12, 1630-1639
- Jainu, M. and Shyamaladevi, C.S. (2005), In vitro and in vivo evalution of free radical scavenging potential of Cissus guandriangularis, Pharma. Biol., 43, 773-779.
- Malpure, P.P., Shah, A.S. and Juvekar, A.R. (2006), Antioxidant and anti-inflammatory activity of extract obtained from Aspergillus candidus MTCC 2202 broth filterate, Ind. J. Exp. Biol., 44, 468-473.
- Mensor, L.L., Menezes, F.S., Leitao, G.G., Reis, A.S, Dossantos, T., Coubes, C.S. and Leitao, S.G. (2001), Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method, Phytother. Res,, 15, 127- 130.
- Geetha, T., Marhotra, V., Chopra, K. and Kaun, I.D. (2005), Antimutagenic and antioxidant activity of quercetin, Ind. J. Expt. Biol., 43, 61-67.
- Gulcin, I. Kufrevioglu, O.I., Oktay, M. and Buyukokuroglu, M.E. (2004), Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica diocia L.), J. Ethnopharmacol., 90, 205-215.
- Winterbourn, C.C., Hawkins, R.E., Brain, M. and Carrel, R.W. (1975), The estimation of red cell superoxide dismutase activity, J. Lab. Clin. Med., 85, 337-341.
- Valentao P, Fernandes E, Carvello F, Betle AP, Sebra RM and Bastos ML (2003) Hydroxy radical and hypochlorous acid scavenging activity of small centaury (Centarium erythraea) infusion, A comparative study with green tea (Camellia sinensis), Phytomed., 10, 517-522.
- Yen, G. and Duh, P. (1994), Scavenging effect of methanolic extract of peanut hulls on free radical and active oxygen species, J. Agr. Food. Chem., 42, 629-632.

This work is licensed under a Creative Commons Attribution 4.0 International License.





