How to Cite | Publication History | PlumX Article Matrix
Sulphoxidation of a Drug Intermediate Using Microorganisms
S. R. Brahmani Priyadarshini1*, Gopal Mugeraya2, M. S. Sandhyavali1 and Anupam Kumar Mishra1
1Dayananda Sagar College Of Pharmacy, Kumaraswamy Layout, Bangalore - 78 India.
2National Institute of Technology karnataka, Surathkal India.
Corresponding Author E-mail: priya.srb@gmail.com
ABSTRACT:
The sulphoxide group is present in various pharmacologically active agents. Recently, there has been an increased interest in the development of methodologies for the preparation of chiral sulphoxide. One of the approaches for the preparation of chiral sulphoxide is to exploit the enzymatic capacity of the microbial cells in bringing about stereospecific sulphoxidation of the achiral sulphide. In the current work, an attempt has been made to screen certain selected microorganisms for sulphoxidation of omeprazole intermediate. Bio-oxidation was performed in phosphate buffer of pH 7.6 with the resting cells of various fungi. The sulphide intermediate (concentration 1g/L) was incubated with the resting cells for 48 h at 30 °C, 160 rpm in a rotary shaker. The work up consisted simply of filtration to remove the spent mycelium, extraction of the filtrate with alkaline dichloromethane and further concentration by evaporation to get the oxidized product. Ten different species of fungi were tested for sulphoxidation, of which only two species, Aspergillus niger and Rhizopus stolonifer, showed conversion. The product was identified by TLC and LCMS, quantification was done by HPLC.
KEYWORDS:
Sulphoxidation; Omperazole; Aspergillus niger; Rhizopus stolonifer.
Download this article as:| Copy the following to cite this article: Priyadarshinopali S. R. B, Mugeraya G, Sandhyavali M. S, Mishra A. K, Sulphoxidation of a Drug Intermediate Using Microorganisms. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Priyadarshinopali S. R. B, Mugeraya G, Sandhyavali M. S, Mishra A. K, Sulphoxidation of a Drug Intermediate Using Microorganisms. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9754 |
Introduction
A number of sulphur compounds are known in which the sulphur is bonded to three substituents and also retain a lone pair of electrons. These types of compounds can in suitable cases exhibit chirality. Sulphoxides also have a structure with three different substituents and a lone pair of electrons around a sulphur atom and so can exhibit chirality1.
Sulphoxides have been obtained in optically active forms by resolution of the racemates. However, optically active sulphoxide have also been prepared by asymmetric synthesis, which are carried out by oxidation of unsymmetrical substituted sulphide. Sulphoxidation in chiral environments is either limited to specific substituents or gives only moderate to low enantiomeric excess of product and use of chiral oxidants can be similarly unrewarding2.
The asymmetric oxidation of a prochiral sulphide is undoubtedly a more direct and commercial method for the synthesis of sulphoxide than the kinetic resolution of a reacemic sulphoxide. The transformations with metal catalyst or isolated enzymes are tedious and expensive. Asymmetric sulfoxidation by whole cells are much cheaper and more convenient, as it does not require expensive co-factors3.
One of the earliest methods of preparing chiral sulphoxides, microbial biotransformation, has not been extensively adopted for synthetic purposes. Despite the proven success of this method in performing stereospecific asymmetric oxidation of certain prochiral sulphides and the associated developments in the application of isolated oxidase enzyme methodology, further work is still necessary to identify new substrates and specific microorganisms which will prove useful for the production of chiral sulphoxides.
The use of enzymes as reagents in organic synthesis provides exciting opportunities for the exercise of regio and stereoselectivity. In case of oxidative reactions catalyzed by oxygenase enzymes, whole microbial cells in an actively growing or resting stage are commonly employed4,5,6,7. In general, organic sulfides, along with other xenobiotic organic compounds are oxidized both in vivo and in vitro by either one or both of two types of mono oxygenases enzymes, those dependent upon cytochrome P-450 for activation and transfer and other using a flavin molecule for the purpose2. It has generally been assumed that the fungal enzymes responsible for oxidation of sulphide to sulphoxide are cytochrome P-450 containing monooxygenases.
some selected fungi were screened for their capacity to carry out sulphoxidation of 5- Methoxy-2-[[(4-methoxy –3, 5-dimethyl-2-pyridinyl) methyl]-sulphinyl]-1 H benzimidazole to omeprazole.
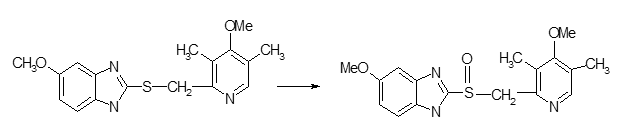
The sulphide selected for screening is an intermediate in the synthesis of omeprazole, a potent proton pump inhibitor. It acts as an inhibitor of gastric H+, K+ ATPase, the gastric acid pump. This being sulphoxide, has an asymmetric center in the sulphur atom and exists in two optical isomers. The S isomer is the desirable sulphoxide for the observed clinical activity.
Fungi selected for screening are Aspergillus niger MTCC 961, Pichia farinosa MTCC 246, Saccharomyces cerevisiae MTCC 174, Candida vishwanathii MTCC 1629, Rhizopus stolonifer MTCC 2198, Aspergillus niger,Aspergillus flavus, Aspergillus oryzae, pencillium species, (All isolated from soil) and bakers yeast.
Materials and Methods
Microorganisms
Saccharomyces cerevisiae: MTCC 174 was obtained from MTCC, Chandigarh. The organism was maintained on YEPD media containing, yeast
extract 3.0 g, peptone 10.0 g, dextrose 20.0 g, agar 20.0 g and distilled water 1000 ml.
Pichia farinosa MTCC 246 was obtained from MTCC, Chandigarh. The organism was maintained on MYA media containing malt extract 3.0 g, yeast
extract 3.0 g, peptone 5.0 g, dextrose 10.0 g, agar 20.0 g and distilled water 1000 ml.
Aspergillus niger (soil isolate) and Aspergillus niger MTCC 961. The organisms were maintained on MRBA media containing, dextrose 10.0 g, peptone 5.0 g,
potassium dihydrogen phosphate 1.0 g, Magnesium sulphate 0.5 g, Rose Bengal
0.0035 g, Agar 20.0 g, distilled water 1000 ml and streptomycin 0.03 g.
Candida viswanathii MTCC 1629: The organism was obtained from MTCC, Chandigarh and maintained on YEPD medium.
Pencillium species (soil isolate): The organism was maintained on MRBA medium.
Rhizopus stolonifer MTCC 2198: The organism was maintained on MRBA Medium.
Chemicals: All chemicals and solvents were from local suppliers and of analytical grade.
Omeprazole standard was obtained from the industry.
Cultivation of Saccharomyces cerevisiae, Pichia farinosa and Candida viswanathii
The organisms from the slant culture was subcultured into 300ml YEPD medium containing Yeast extract 0.3 g, peptone 1.0 g, dextrose 2.0 g and distilled water 100 ml, pH was adjusted to 7.0 and was sterilized at 121ºC for 15 min. The cultures were grown at 30ºC, 160 rpm for 24 h.
10% volume of the fermentation medium was used for inoculation of 2.5 L of YEPD medium. The inoculated medium was incubated at 30ºC, 160 rpm for 48 h. After 48 h of growth, the cells were separated by filtration using buchner funnel and the biomass was washed with phosphate buffer twice.
Cultivation of Aspergillus niger, Rhizopus stolonifer and Pencillium.
The spores from the maintenance culture were inoculated to, 2 L of potato dextrose medium containing potato 400.0 g, dextrose 20.0 g and distilled water 2000ml.
The pH of the medium was adjusted to 6.0. The medium was sterilized at 121 ºC for 15 min. The inoculated medium was incubated at 25ºC for 5 days to get sufficient biomass. The mycelial biomass was separated by filtration and washed with phosphate buffer twice.
Procedure for Bio-oxidation
10 g of the biomass was taken in 20 ml phosphate buffer of pH 7.6, 20 mg of omeprazole intermediate dissolved in 2 ml alcohol was added to the above suspension and incubated at 30˚C, 160 rpm for 48 h.
The reaction mixture was filtered to remove the biomass. Filtrate was extracted with alkaline methylene dichloride (20 ml x 3), washed with 20 ml of brine twice and dried over anhydrous sodium sulphate. The organic extract was then concentrated by evaporation.
HPLC Analysis
The oxidized product of omeprazole was quantified by HPLC.
Chromatographic condition
Mobile phase : phosphate buffer (pH 7.6) : methanol ( 25:75)
Column: C18 phenomenex
250 x4.6 mm, .5 μm,
Flow rate: 0.8 ml/min.
Wave length: 280 nm
Injection volume: 20 μl.
Standard Preparation
25 mg of the Standard was dissolved in 25 ml of methanol and diluted to get concentration of 2.5 to 10.0 μg.
Sample preparation
The concentrated sample was made up to 10 ml with methanol.
Results and Discussion
Totally ten organisms were selected for carrying out sulphoxidation of omeprazole intermediate (Table – 1), out of which one species of Aspergillus niger showed negligible conversion while Rhizopus stolonifer was found to be effective in bringing about sulphoxidation of the selected substrate. Even though Aspergillus species are known to have good sulphoxidation acitiviy, the above selected species were inactive in general on the omeprazole intermediate.
Table 1
|
Microorganisms
|
Yield in mcg |
|
Aspergillus niger MTCC 961
|
00.00 |
|
Aspergillus niger (soil isolate)
|
13.06 |
|
Aspergillus flavus
|
00.00 |
|
Aspergillus oryzae
|
00.00 |
|
pencillium species
|
00.00 |
|
Pichia farinosa MTCC 246
|
00.00 |
|
Candida viswanathii MTCC 1629
|
00.00 |
|
Saccharomyces cerevisiae: MTCC 174
|
00.00 |
|
Bakers yeast
|
00.00 |
|
Rhizopus stolonifer MTCC 2198
|
138.02 |
The reaction was monitored by TLC (Benzene: ethyl acetate: methanol:: 5:3:1), and the product was confirmed by HPLC and LCMS. The standard for the analysis was obtained from industry.
Maintainence of alkalinity during reaction and extraction process is critical in sulphoxidation of omperazole intermediate as omperazole is unstable in acidic or neutral medium.
References
- Michael North, Principles and Applications of Stereochemistry, Stanely Thomas (publishing) Ltd., United Kingdom, 56 (1998).
- Herbert Leslie Holland, Chiral Sulfoxidation by Biotransformation of Organic Sulfides, Chem.Rev., 88, 473(1988).
- Ai-Tao Li, Jian-Dong Zhang, Jian-He Xu, Wen-Ya Lu and Guo-Qiang Lin, Appl. Environ. Microbiol., 75, 551 (2009).
- European Patent EP0795024
- Herbert Leslie Holland, Frances M. Brown and Brett G. Larsen, Tetrahedron Asymmetry, 6, 1561(1995).
- Kurt Faber, Biotransformations in Organic Chemistry, Springer, New York, 177 (2004).
- Derek R. Boyd, Narain D. Sharma,Simon A. Haughey,Martina A. Kennedy, Brian T. McMurray, Gary N. Sheldrake,Christopher C. R. Allen,Howard Dalton and Kenneth Sproule, J. Chem. Soc., 1, 1929(1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.





