How to Cite | Publication History | PlumX Article Matrix
A Bioactive Terpenoid from the Leaves of Callicarpa Macrophylla Vahl.
Vinay Kumar Verma, N. U. Siddiqui* and Mohd. Aslam
Natural Product Research Division, Post graduate Department of Chemistry, G. F.(P.G.) College (Rohilkhand University), Shahjahanpur - 242 001 India.
Corresponding Author E-mail: vnkmr912@gmail.com
ABSTRACT: From the chloroform extract of the leaves of Callicarpa macrophylla Vahl (Family :- Verbenaceae) two tetracyclic diterpene viz. calliterpenone and its monoacetate were isolated and their structures and stereochemistry were established by 1H & 13C NMRhhhhh spectroscopies.
KEYWORDS: Callicarpa macrophylla; Verbenaceae; calliterpenone and its monoacetate
Download this article as:| Copy the following to cite this article: Verma V K, Siddiqui N. U, Aslam M. A Bioactive Terpenoid from the Leaves of Callicarpa Macrophylla Vahl. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Verma V K, Siddiqui N. U, Aslam M. A Bioactive Terpenoid from the Leaves of Callicarpa Macrophylla Vahl. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9823 |
Introduction
Plant Callicarpa macrophylla Vahl. (Family: Verbenaceae) is an important less known medicinal plant of the lower warm valleys of the Himalaya and is commonly known as Priyangoo or Daya. It is a perennial, deciduous shrub attaining 2.5mt. in hight. Essential oil obtained from different parts of Priyangoo through steam distillation ,revealed oil content in young leaves and tender stem , panicles and seeds while no essential oil content was observed in the roots. All The parts are important and are used to cure many diseases. The bark is used to heal cut and wounds. Seeds and roots are used for digestion and leaves are used in rheumatism. The fruits are used for blisters and boils. Various extract of this plant have shown anti-inflammatory, antifungal and antibacterial activities.1-4
Material and Method
The fresh leaves of Callicarpa macrophylla Vahl. (Family: Verbenaceae) was procured from the Central Institute of Medicinal and Aromatic Plants (CIMAP) Lucknow (U.P.) in Octuber 2009. The plant was identified by a Taxonomist of the center and a specimen was kept for record.
The air dried and coarsely powered leaves (3kg) was sequentialy extracted with petroleum ether (600 -800), chloroform and methanol by the soxhlet apparatus (6 times x 1L. each).The fraction of each extract were mixed together and the excess of solvent was evaporated under reduced pressure. Out of these extracts only chloroform extract was considered for further examination. A column of silica gel was prepared well stirred with petroleum ether. Than a slarry of chloroform extract (8gm) was made and digested over this column. The column was eluted with different solvents like petroleum ether, benzene, chloroform, ethyl acetate, acetone, methanol and their mixtures of increasing polarity. From the eluent two compounds could be separated. A compound was obtained by eluting benzene-chloroform (8:2). The compound was further purified by crystallization. The compound was the characterized by m.p., solubility and different spectral studies like IR, NMR( 1H &13C hhhhh).
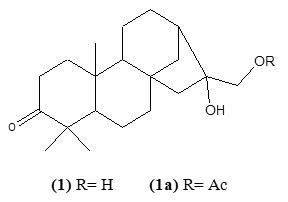
The compound crystallized from chloroform as white needles, m.p.- 1560C and was characterized as calliterpenone and its monoacetate by comparing spectral data 1H NMR (CDCl3) δ 3.64 (2H, H-17), δ 2.31(2H, H-2), δ 1.21 – 1.10 (3H, 3xCH3 , 18,19,20); 13C NMR (CDCl3) δ 32.9(C-1), δ 33.9(C-2), δ 213.4(C-3), δ 50.7(C-4), δ 49.0(C-5), δ 22.1(C-6), δ 32.0(C-7), δ 31.2(C-8), δ 50.7(C-9), δ 29.4(C-10), δ 20.5(C-11), δ 21.8(C-12), δ 40.6(C-13), δ 32.3(C-14), δ 40.6(C-15), δ 76.8(C-16), δ 72.1(C-17), δ 18.9(C-18), δ 18.9(C-19), δ 20.5(C-20).
Result and Discussion
In the 1H NMR spectrum of the compound (1) a two proton downfield signal at δ 3.64 is assignable to methylene proton H-17 to which a hydroxyl group is attached. Another downfield signal at δ 2.31 was assigned to H-2 proton next to carbonyl function. The methyl proton signals resonates in the rang δ 1.21- δ .10.
The13 C NMR spectrum of the compound clearly indicates twenty signals which corresponds to its molecular formula C20H32O3. In its 13C NMR spectrum a downfield signal at 213.4 was clearly indicated the carbonyl group at C-3. Another downfield signals at 72.1 and 76.8 were assigned to C-17 and C-16 to which hydroxy group is attached. On the basis of above finding the structure of the compound was characterized as Calliterpenone.
In its acetate (1a) an additional signal for acetyl proton in its 1H NMR appeared at δ 2.09. The rest of the signals are almost same except the signal of methylene proton H-17 which was slightly shifted in downfield region. In the 13C NMR spectrum of the compound(1a) an additional signals at δ 171.0 was reasonably designated to acetoxy carbon 5-8.
Acknowledgement
The authors are thankful to the Principal, G.F.College and Head , Department of Chemistry for providing facilities for carrying out this work.
References
- Chopra R.N., Nayar S.L. and Chopra I.C., Gloss. Ind. Med. Plant, CSIR ,PID,
- Delhi (1956)
- Kirtikar K.R. and Basu B.D., Indian Medicinal Plants,Dehradoon(1984) 3. Verma R.K., Singh A.K., Srivastava P., Shanker K., Kalra A. and Gupta M.M.,
- Liquid Chromatography & Related Technologies, 32, 2437-2450 (2009)
- Sharma A.K., Negi K.S., Bhandari D.C., Shukla Y.H. and Pareek S.K., ENVIS Bulletin Vol. 12(1) : Himalayan Ecology.
- Chatterjee A., Desmukh S.K. and Chandrasekharan S., Tetrahedron, 28, 4319- 4323(1972)
- Ahmad S.A. and Zaman A., Tetrahedron Letters, 24, 2179-2182(1973)
- Subramanian S.S., Nair A.G.R. and Vedantham T.N.C., Phytochemistry, 13,306-307(1974)
- Fujita E., Ochiai M., Ichida I., Chatterjee A. and Desmukh S.K., Phytochemistry,14, 2249-2251(1975).

This work is licensed under a Creative Commons Attribution 4.0 International License.





