How to Cite | Publication History | PlumX Article Matrix
Anatomy of Three Piper Nigrum Genotypes With Respect To Phytophthora Foot Rot Disease
Remmia Raghavan1, S. Elumalai1, K. Nirmal babu2 and P. Jayaraman3
1P.G. Research Department of Plant Biology & Biotechnology, Presidency College, Chennai - 600 065 India.
2Department of Crop Improvement & Biotechnology Indian Institute of Spices, Research, Calicut, Kerala - 673 012 India.
3Plant anatomy Research Centre, No.4 IInd Street, Shakthi Nagar, West Thambaram, Chennai - 670 045 India.
ABSTRACT: Foot rot caused by phytophthora capsici is the most serious diseases of black pepper. Though all varieties of black pepper are susceptible to this pathogen, variation do exist concering the degree of tolerance and mechanisms of defense. Anatomical features such as thicker epidermis, smaller no: of epidermal appendages, smaller cortical cells, smaller stele, thicker pericycle cells and smaller vascular bundles and compact arrangement of cells can be attributed to immunity of II -SR Shakthi to phytophthora capsici as compared to kalluvally. Observed anatomical differencess between the 3 genotypes can be used for selecting parents for disease resistant breeding .
KEYWORDS: Anatomy; piper nigrum; phytophthora capsici; Disease Resistance
Download this article as:| Copy the following to cite this article: Raghavan R, Elumalai S, Babu K. N, Jayaraman P. Anatomy of Three Piper Nigrum Genotypes With Respect To Phytophthora Foot Rot Disease. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Raghavan R, Elumalai S, Babu K. N, Jayaraman P. Anatomy of Three Piper Nigrum Genotypes With Respect To Phytophthora Foot Rot Disease. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9653 |
Introduction
Black Pepper is affected by several diseases caused by Fungi, Bacteria, Virus and Mycoplasma, besides nutritional disorder. Crop losses due to diseases and pests are identified as major causes of low productivity of pepper in India (Sarma and Anandraj 1995). The earlier record of diseases of pepper in India was that of Barber (1903, 1905). Butler (1906) also recorded the death of pepper and Rao (1929) isolated phytophthora from diseased pepper Phytophthora capsici occurs on all parts of the plant and cause severe economic damage. The symptoms expression depends upon the site of infection and extend of damage (Mammooty 1978, Anandraj and Sarma, 1995). Aerial infection occurs on the summer shoots, foliage, spikes and branches causing blight, spike shedding, defoliation and die back and at times death of the plants. Infection on the runner shoots often reach the collar causing foot rot. There is no effective control medicine to combat this malady.However, IISR Shakthi ( Released from IISR, Calicut ) is reported to be resistant to phythophthora capsici while black pepper cultivars panniyur-1 and kaluvally reported to be susceptible & tolerant respectively to the disease ( Kuch & Khew , 1982 and Sharma Nambiar ,1982 ) Hence, the present study was aimed at analyzing anatomical basis of disease resistant among susceptible,tolerant and resistant genotypes of black pepper to phytophthora capsici.
Materials and Methods
The plant specimens for the proposed study (IISR Shakthi, Panniyur -1, Kalluvally) were collected from IISR Experimental Farm, peruvanamuzhi, Calicut, Kerala. Three varieties of P. nigrum VIZ, Panniyur-1 (susceptible) Kalluvally ( Tolerant ) I I SR Shakthi (Resistant) were selected for the study. Rooted cuttings grown in polybags under green house condition (4 month old) were used. Pure culture of P. Capsici isolated from infected black pepper leaves were inoculated on carrot agar medium in petriplates and incubated at 270 C for optimum growth. Seven day old cultures having profuse growth were used for inoculation . Culture discs of uniform size (5mm dia) were applied on the ventral side of the leaf lamina, stem, petiole after making pin pricks (10 no:s) at the points of contacts. The Mycellium was crushed with the mortar and pestle and was inoculated into soil. The Resistant, susceptible,Infected plant materials were cut and fixed in FAA.(Formalin 5 ml + Acetic acid 5ml + 70% Ethyl Alcohol – 90ml).After 24 hrs of fixing, the specimens were dehydrated with graded series of tertiary -Butyl alcohol as per the schedule given by Sass, (1940). Infiltration of specimens was carried by gradual addition of paraffin wax (melting point (58 – 600) until TBA solution attained super saturation. The specimens were cut into paraffin Blocks, which were sectioned with the half of Rotary Microtome. The sections were stained with Toluidine blue as per the method published by O’ Brien et al (1964). Microscopic descriptions of tissues are supplemented with Micrographs. Photographs of different Magnification were taken with NIKON LABPHOTO2 Microscopic units. Magnifications of the figures are indicated by scale bars. Descriptive terms of the anatomical features are given in the standard Anatomy Books (Esau, 1964).
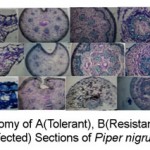 |
Figure 1
|
Table 1: Comparison Of Anatomical Features Of Resistant and Tolerant Variety Resistant Variety Tolerant Variety
| Parts | Resistant Variety | Tolerant Variety | ||
| I.
|
LEAF
|
Thickness of the midrib
Cuticle of the midrib (adaxial side) Cuticle of the abaxial side Adaxial epidemis thicknees Abaxialepidemis thickness. Leaf palisade tissue height Palisade cells Spongy mesophyll tissue Spongy mesophyll tissue |
650µm thick
5 µm thick 8 µm thick 12µm thick 10 µm thick 30 µm Compact 2.3 layered
Compact
|
720 µm thick
very thin very thin 20 µm 7 µm thick 20 µm loosely arranged layered
loosely arranged
|
|
|
Ground tissue of the petiole ( Angolan, compact, thick walled)
Sclerenchymatous bundle laps wide & thick walled |
Ground tissue with circular cells, less compact & this walled.
Bundle caps- narrow and thin walled |
||
|
STEM: epidermal layer – 25 µm thick & cuticle – 8 µm thick
Hypodermal scteremchyma. Cyliader – 100 µm thick; cells thick walled with narrow lumen.
Corticl zone narrow – 50 µm wide, with small, compact cells.
Outer vascular bundles more in no:, large in size (24 bundles; 150 x 250 xylem – Sclerenchyma and thick walled vessels.
Cortical sclerenchymma cylinder 5 or 6 layered, cells with walls
Inner (central) vascular bundles larger, 300 x 350 µm thick, vessels 70mm wide,
ROOT:- The rhizodermis with subcised outer walls is well preserved; the cortical zone is narrow with fair thick walled compact cells. The stele has wide thick walled Xylem elements as well as thick walled fibres. |
Epidermal layer 20 µm thick cuticle – 5 µm thick.
Hypodermal sclerenchyma cylinder – 90 µm thick, cells wide, walls loss thick.
Cortical zone – 100 µm wide, cortical cells wide & thin walled.
Outer vascular bundles less in no: (about 15 bundles) smaller in size (100 x150 µm m)., with parenchymatons cells in the bundle and thin walled vessels.
Corticl sclernchyma cylinder 3 layered, cells – thin walled.
Central vascular bundles smaller in size, 250 x 350 µm thick, vessels 40 µm wide.
The rhizodermis bears close root hairs, the cortex is wide, cells having thin walls and less compact. The stele consists of narrow, thin walled scattered xylem elements & dense xylem fibres.
|
Structural Changes of the Infected Organs of Pepper
Leaf
The infected leaf exhibits thick continuous layers. Cleistocarps, surrounded all around by thick dark covering. The cleistocarps are in different stages of development, they are on the adaxial surface of the leaf. In some of the first bodies, there is an apical pole through which conidia are likely to be released. This conidiophores are thicker and dense. The conidia are elliptical and elongated. The subepidermal cells break from the epidemis and become crumbled. The mesophyll tissues are also distorted due to the formation of the first bodies on the epidemis.
Petiole
The infection occurs in the adaxial groove of the petiole. The lateral borders of the groove are wounded. The cells along the borders undergo wound healing process by producing this zone of small suberised cells. This infection seems to penetrate the cells along the concavity so that some of the cells are crushed and others become dark and compressed. Since the infected is in initial stage, interior tissue and vascular strands are not altered significantly.
Stem
In a young stem, the critical zone consists of only parenchymatous tissue and no sclerenchyma cylinder is seem. So the inoculated pathogen has spread into wide corticial tissue, which become dark and crushed. This stimulates poliferation and hyperplasia of the inner cortical cells adjacent to vascular bundles.
In the old stem, where there is a cortical cylinder of sclerenchyma cells, the entry of the pathogen occurs by breaking sclerenchyma cylinder and later infection spreads to the outer cortical tissues. The fungal pathogen seems to be intra cellular. The fungal organism is found occluding the cell cavity of the cortex.
During advanced stage of infection, the critical paramchyma cells on the pith, in the medullary rays, cortex and within the vascular bundles get collapsed into dark amorphous structures. However, there is no evidence of presence of fungal pathogen to the xylem elements of sclerenchyma cells.
Roots
In the root entry of the pathogen is through an injury made in the cortical region. It spreads in the cortical cells and then enters into the stele. More and more cortical cells become invaded by the pathogen and the cells are turned into dark structure. The most obvious effects of the pathogen is manifested in the xylem elements. The xylem elements are occluded by dark, amorphous substances which evidently blocks the flow of water to the aerial system of the plant.
Conclusion
The comparative account of the anatomical features of resistant variety and susceptible variety reveals certain structural differences between these two. Previous studies by Marks and Mitchell (1971), Miller and Maxwell (1984), Philips etal (1987), Hamalavi etal (1995), and Markose (1996), on various host plant infested by phytophthora have established that thicker epldermis, smaller no: of epidermal appendages, smaller cortical cells, smaller stele, thicker pericyclic cells, vascular bundles and compact arrangement of cells can be attributed to phytophthora infection.
In the present study we have obtained results similar to those mentioned above in the resistant variety of piper nigrum as compared to susceptible variety . physical barriers found in the resistant variety seams to play a pivotal role in resisting fungal infestation. That it can be concluded that anatomical differences observed in 3 piper nigrum genotypes can be used for screening various genotypes against tolerance / resistant to phytophthora capsici The selection of tolerant / immune genotype via screening will be helpful to evolve resistant / tolerant plant and also on the selection of plants for further breeding.
Acknowlwdgement
This paper forms a part of PhD thesis ( Plant biology & Biotechnology submitted by Miss Remmia Raghavan to University of Madras, Nov 2010. Deeply acknowledged to the Director IISR experimental form Peruvanamuzhi Calicut, Kerala for providing the plant materials for study.
References
- Anandaraj M. Sharma, Y.R (1995) Diseases of black Pepper (Piper L.) and their management. J. Spices and Aromatic Crops, 4, 17-23.
- C.A., (1903) Pepper diseases in the Waynad. Trop. Agric., (Colombo) 22, 805-807.
- R.J. (1906) The wilt disease of Pigeon pea and Pepper. Agric. J. India, 1,25-26.
- K., (1964) Plant Anatomy John Wiley and Sons. New York. pp. 767. Easu. K., (1979). Anatomy of seed Plants. John Wiley and Sons. New York.
- Z.A., J.A. Menge and F. B. Guillemot (1995) . Infection court and factors affecting the expansion of stem canker of avocado caused by Phytophthora citracola. Pl. Dis. 79: 384- 88
- T.K. and K.L. Khew (1980). A screening technique useful in selecting for resistance to black pepper Pyytophthora palmivora. Malayasian Agri. J. 54: 39-45.
- B.L., (1996) Genetic and blochemical bases of resistance to bacterial wilt in chilli. Ph. D. (Hort.) thesis, Kerala Agricultural University, Thrissur, Kerala
- G.C., and J.E. Mitchell. (1971). Penetration and infection of alfalfa roots by Phytophthora megasperma and pathological anatomy of Infected roots. Canadian. J. Bot. 49: 63-67.
- S.A. and D.P. Maxwell (1984). Light microscopic observations of susceptiable, host resistant and nonhost resistant interactions of alfalfa with Phytophthora megasporma. Canadian J. Bot 62: 109-116.
- K.P., (1978) Quick wilt diseases of pepper (Piper nigrum Linn.) -1. Symptomatological studies on the quick wilt diseases of pepper. M.Sc. (Agri,) Thesis, Kerala Agri. University, pp. 87
- O’Brien. T.P., Feder. N. and Mc. Cull. M.E. (1964) Polychromatic Staining of Plant Cell walls by toludine blue- O. Protoplasma; 59: 364-373.
- D. B.R. Grant and G. Weste. (1987). Histological changes in the roots of an avocado cultivar Duke-7. Infected with Phytophthora cinnamont. Phytopathology. 77. 691-698.
- V.M.K., (1929) Annual Report for 1927-28, Department of Agri., Mysore pp.19.
- Y.R. and K.K.N. Nambiar (1982) Foot rot disease of black pepper (Piper nigrum L.) Proceedings of the Workshop on Phytophthora Diseases of Tropical Cultivated Plants. (Nambiar. K.K.N.) CPCRI, Kasargode, Kerala, pp. 209-224.

This work is licensed under a Creative Commons Attribution 4.0 International License.





