How to Cite | Publication History | PlumX Article Matrix
Antagonistic Activity Of Pseudomonas Isolates From Kolli Hills Against Fusarium Aphanidermatum
Nagaraja Suryadevara1,2*, P. Ponmurugan3, V. Kanimozhi1,2 and K. Manju3
1Research & Development Department, Bharathiyar University, Coimbatore (India). 2Vivekananda College of Arts and Sciences for Women, Thiruchengode (India). 3Department of Biotechnology, K.S.R. College of Technology, Thiruchengode (India).
ABSTRACT: Plants Growth Promoting Rhizospheric bacteria (PGPR) are capable of increasing directly or indirectly crop yield. Pseudomonas has been found in large populations, which inhabit in the rhizosphere of several agriculture crops. The aim of this study was to determine the antagonistic activity in vitro and in vivo of Kolli Hill Pseudomonas (KLP) Isolates of plant growth promoting rhizobacteria against Fusarium aphanidermatum, and to assess their ability to produce IAA and to promote plant growth. The bacterial strains were tested to assess their fungal growth inhibition and plant growth stimulation. The plant growth assays were done in a modified microcosm experiment and in greenhouse conditions. The results demonstrated that these rhizobacteria produce IAA and exhibit antagonistic effect on Fusarium aphanidermatum inoculated in the substrate. Pseudomonas Sps out for their plant growth stimulation in maize and for the biological control exerted on Fusarium aphanidermatum. The strains of pseudomonas Sps. KLP6, KLP7 and KLP11 showed the best results in disease suppression, which was achieved up to 80%. These results have practical relevance, since potentially effective bacterial strains were selected and could be used in agriculture, for the enhancement of plant growth and disease control in maize crop.
KEYWORDS: Fusarium aphanidermatum; pseudomonas; Indole Acetic Acid (IAA); KLP
Download this article as:| Copy the following to cite this article: Suryadevara N, Ponmurugan P, Kanimozhi V, Manju K. Antagonistic Activity Of Pseudomonas Isolates From Kolli Hills Against Fusarium Aphanidermatum. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Suryadevara N, Ponmurugan P, Kanimozhi V, Manju K. Antagonistic Activity Of Pseudomonas Isolates From Kolli Hills Against Fusarium Aphanidermatum. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9227 |
Introduction
Microorganisms that colonize the rhizosphere can be classified according to their effects on plants and the way they interact with plant roots: some of these microorganisms are plant pathogens whereas others trigger beneficial effects (Mantelin and Touraine, 2004; Matiru and Dakora, 2004). In the rhizosphere, bacteria are abundantly present, more often organized in microcolonies. Some of these rhizobacteria not only take advantage of the nutrients secreted by the plant roots but also have a positive effect on the plant in a direct or indirect way, resulting in a stimulation of its growth (Bloemberg and Lugtenberg, 2001).Plants growth promoting bacteria (PGPB) are usually classified into two groups according to whether they affect plant growth indirectly or directly and are referred to as biocontrol-PGPR and PGPR, respectively (Bashan and Holguin, 1998). Indirect effects are related to production of metabolites such as antibiotics, siderophores, or cyanhidric acid (HCN), that decrease the growth of phytopathogens and other deleterious microorganisms. Direct effects are dependent on production of plant growth regulators, or improvements in plant nutrient uptake (Glick, 1995; Myoungsu et al., 2005).
Materials and Methodology
Isolation and biochemical characterization of isolates of Pseudomonas
Rhizospheric soils of different agronomic parts of kollihills in Nammakal TN, India were collected from October to December, 2008, for the isolation of Pseudomonas Spp. Pseudomonas isolates were isolated from the soil on nutrient agar medium or King’s medium as per the standard method (Stein A, Fortin JA, Vallee G.1990). Each isolate showing characteristic growth, pigmentation and biochemical reactions as described in Bergey’s Manual of Determinative Bacteriology for Pseudomonas and related species was purified and given an isolate number. Microbiological media were purchased from Hi-Media lab. Pvt. Mumbai, India
All the 12 isolates of Pseudomonas were biochemically characterized for Gram reaction, carbohydrate fermentation, H2S production, NO3-reduction, IMViC tests, oxidase test, starch hydrolysis, and gelatin liquefaction as per the standard methods (Holt JG, Krieg NR, 1994).
Detection of indole-3-acetic acid (IAA)
IAA production was determined by Salkowski’s method and Thin-Layer Chromatography (TLC). The bacteria were cultured in King’s B liquid medium supplemented with tryptophan (100 mg/L) for 24 h at 30 0C till the stationary phase of growth. Supernatants were obtained after centrifugation of cell cultures at 8000rpm for 5 min. The control was uninoculated culture medium. Two milliliters of Salkowski reagent (Sarwar et al., 1992) were added to 1 mL of culture supernatant in a test tube and incubated at 25 0 C for 30 min. The optical density of the solutions was quantified using a Genesys 20 Spectrophotometer (ThermoSpectronic) at 533 nm. Thin-layer chromatography (TLC) was performed on silica gel-covered glass using EIA solvent: Ethylacetate/ Isopropanol/Ammonium (45:35:20, vol/vol). The chromatograms were developed by spraying with Salkowski’s reagent followed by heating at 2000C. Five replicas were established for each strain and all the experiments were repeated three times.
Extraction of crude IAA
Single bacterial colonies of 3 isolates of Pseudomonas spp. (KLP6, KLP9 and KLP10) were inoculated in 200 ml of nutrient broth and incubated at 28 ± 2 oC for 1 week on a shaker incubator. Bacterial cells were separated from the supernatant by centrifugation at 10,000 rpm for 30 min. The supernatant was acidified to pH 2.5 to 3.0 with 1 N HCl and extracted twice with ethyl acetate at double the volume of the supernatant. Extracted ethyl acetate fraction was evaporated to dryness in a dessicator. The extract was dissolved in 300 ml of methanol and kept at -20 oC.
In vitro antagonism test
To determine the antagonistic effect in vitro with Fusarium aphanidermatumwas cultured in potato dextrose agar (PDA) for 5 d at 30 0 C and the bacterial strains in King’s B liquid medium for 24 h at 30 0 C. Then 100 µL of each bacterial culture was spread on 9-cm-diameter Petri dishes containing King’s B Agar and immediately after this, 5-mm plugs from the leading edge of a 5-days-old fungal culture were placed in the centre of each Petri dish. The control culture was set inoculating the fungus in King’s B Agar not inoculated with bacteria. The plates were incubated for 7 d at 30 0C. The fungal inhibition was scored by measuring radial growth of the fungus in mm in every plate and the inhibition percentage was calculated through a comparison with the control plate, according to Bashan et al. (1996). The experiment was repeated three times with five replicates per treatment.
Plant growth promotion assay
Maize seeds () were used in this study. For disinfection, the seeds were immersed for 5 min in a Tween 80 solution (250 seeds/200 mL), for 1 min in 70% ethanol and then for 20 min in a 5% filtered calcium hypochlorite solution with Tween 80. After the last immersion the seeds were rinsed 4 times with sterile water. Some seeds were placed in a nutrient agar plate to confirm the disinfection process. The bacterial strains were cultured in King’s B liquid medium at 30 8C for 24 h (250 rpm). The concentration was adjusted to 108 cfu mL_1 using the Neubauer slide. The resulting broth was used as inoculum. After surface disinfection, the seeds were submerged for 45 min in the bacterial inoculum, which were considered as treatments and sterile King’s B culture medium as negative control. After incubation, excess inoculum w as removed and seeds were immediately planted. The test was carried out following a modified microcosm experiment (Kabir et al., 1995), for 12 d at 30 _ 2 8C. The treated seeds were placed in pots containing sterile Rhodic Nitisol soil. This soil was collected from the upper 35-cm of the soil profile and air dried for approximately 10 d. Prior to drying the soil was ground and sieved through a 0.5-cm mesh screen, sterilized as described by Herna´ndez et al. (1995) and kept at 25 0C before use. The shoot length (cm), longest root length (cm), shoot fresh weight (g) and root fresh weight (g) were measured. The experiment was repeated three times with four replicates per treatment.
In vivo antagonism test
This assay was carried out in maize plants in order to prove the inhibitory effect of the strains on Fusarium aphanidermatumin vivo. The fungal inoculum was prepared from potato dextrose broth (PDB) cultures incubated at 30 0C for 7 d at 250 rpm, and then the propagules were washed and centrifuged three times with sterile saline solution. Part of the soil was infested by mixing it thoroughly with the fungal inocula adjusted to a final concentration of 107 propagules per gram of soil. The bacterial inocula were prepared and inoculated on the seeds as described above. Then the following treatments were established: T1, uninoculated seeds in uninfested soil; T2, uninoculated seeds in fungus-infested soil; from T3 to T6, seeds inoculated with the effective bacterial inocula in uninfested soil; T7– T10, seeds inoculated with the bacterial inocula in the fungus infested soil. The test was carried out following a modified microcosm experiment (Kabir et al., 1995). The antagonistic effect was evaluated 21 days after seeds germination, measuring the shoot and root length (cm), shoots and root fresh weight (g), the number of leaves per plant and the percentage of plants showing disease symptoms. A scale from 1 to 7 was used to assess the percentage of plants showing disease symptoms: scale 1, no symptoms showed; scale 3, symptoms were shown in the roots up to 5%; scale 5, symptoms were shown in the roots up to 25%; scale 7, more than 25% of the roots showed disease symptoms and the seeds were severely damaged. A completely randomized design was established with three replicates (three plants per replicates) per treatment and three repetitions of the experiment.
Results and Discussions
A total of 12 isolates Pseudomonas spp. were isolated from rhizospheric soil and tentatively identified on the basis of biochemical tests and sugar fermentation behavior as described in Bergy’s Manual of Determinative Bacteriology. These bacterial isolates were screened for their ability to produce plant growth regulator, IAA. Varying levels of IAA production were recorded (Table 1)
Table 1. IAA production by Pseudomonas isolates after 7 days of incubation.
| S.No | Isolate Number | IAA production (µg/ml) |
| 1 | KLP1 | 22.40 |
| 2 | KLP2 | 18.00 |
| 3 | KLP3 | 13.40 |
| 4 | KLP4 | 16.00 |
| 5 | KLP5 | 16.50 |
| 6 | KLP6 | 43.20 |
| 7 | KLP7 | 21.40 |
| 8 | KLP8 | 18.50 |
| 9 | KLP9 | 35.60 |
| 10 | KLP10 | 31.00 |
| 11 | KLP11 | 25.20 |
| 12 | KLP12 | 13.50 |
All 12 Pseudomonas isolates were able to produce IAA in tryptophan medium in the range 13.40 to 43.20 µg/ml(Table.1 ). The strains like KLP6, KLP9and KLP10 produced in respect to 43.20, 35.60 and 31.00 µg/ml. our test isolates showed a similar level of IAA production to those recorded by other researchers (Farah Ahmad et.al, 2004).
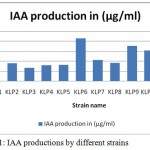 |
Figure 1: IAA productions by different strains.
|
Antagonistic nature
This test showed the antagonistic effect exerted on the phytopathogen F. phythium by the pseudomonas strains (Fig. 2), although not all of them displayed the same competence. The best performing strains were KLP6, KLP7 and KLP11, within the range from 84.0% to 34.0% of fungal growth inhibition.
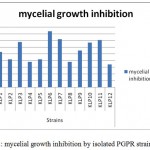 |
Figure 2: mycelial growth inhibition by isolated PGPR strains.
|
In vivo antagonism test
The in vivo experiments demonstrated the stimulation and the biological control effects of the Pseudomonades in maize crop. Only four of the effective strains KLP06, KLP7, KLP11 and KLP 03 showed efficient control in vivo of A. pythium. The disease symptoms were suppressed from 66.7% to 88.9% when pseudomonades were inoculated in plants grown in fungus-infested soil. Most symptomatic roots were found in the scales 3 and 5, showing disease symptoms of 1–5% approximately. Fungal suppression was based on observations of symptoms of fungal disease in the roots. Furthermore, the shoot length, shoot and root fresh weight were increased in all the plants treated with bacteria, compared to the nonfungus- inoculated and fungus-inoculated controls. Significant differences were found in the number of leaves per plant between the fungus-inoculated control and the other treatments.
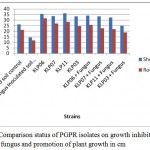 |
Figure 3 Comparison status of PGPR isolates on growth inhibition of fungus and promotion of plant growth in cm.
|
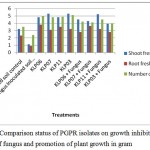 |
Figure 4: Comparison status of PGPR isolates on growth inhibition of fungus and promotion of plant growth in gram. |
Plant growth promotion assay
Plant growth was measured and the effect of bacterial inoculation on the plant was assessed (Fig.5). The shoot length data showed significant differences for all the treatments versus the control. The strains that promoted greater plant growth were KLP3, KLP6 and KLP11. The shoot and root fresh weights were increased by the treatment with KLP6,KLP9, KLP10 and KLP8, but the best performing strains were KLP3,KLP6 and KLP7, respectively. The length of the root was boosted when the seeds were inoculated with KLP3 and KLP9.
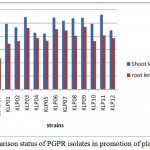 |
Figure 5 Comparison status of PGPR isolates in promotion of plant growth in cm.
|
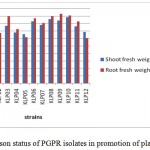 |
Figure 6: Comparison status of PGPR isolates in promotion of plant growth in grams.
|
Discussion
The effectiveness of plant growth promoting rhizobacteria for the biocontrol of phytopathogens has been proved in others studies (Bashan and de-Bashan, 2002; Berg et al., 2005; Compant et al., 2005; Kenneth et al., 2006; Hernandez-Rodrıguez et al., 2008). This research has revealed that these Kollihills rhizobacterial strains are capable of promoting plant growth in maize, although only three of them (KLP6, KLP7 and KLP11) are able to control F. phythium in vivo.
Plant growth promotion was assessed and all of the strains showed beneficial effects in maize plants. These results could be due to the production of indole acetic acid (IAA) by the rhizobacteria, although the IAA concentrations were low compared to other bacterial genera, such as Azospirillum sp. (Bashan et al., 1996) and Acetobacter sp. (Bastian et al., 1998). Our test isolates KLP6, KLP9and KLP10 showed a similar level of IAA production to those recorded by other researchers (Farah Ahmad et.al, 2004).
Similar results have been reported, showing that the inoculation of maize plants with rhizobacteria increases growth and the yield of the plant (Mao et al., 1997; Egamberdiyeva, 2007; Hernandez-Rodrıguez et al., 2008). The IAA is capable of inducing the enlargement of the plant root system which enhances the absorption of nutrients and water and thus, increases effectively plant growth (Mantelin and Touraine, 2004). The production of plant growth substances by PGPR has been reported to be an important trait for their survival. Additionally, root colonization by PGPR may induce the production of plant growth substances by the plant (Patten and Glick, 2002). The control of F. phythium was achieved by the strains tested in vitro and in vivo. All the strains showed antagonistic ability in vitro. These results could be due to the combination of some bacterial traits for the control of phytopathogens, such as the competition for nutrients and the production of biocontrol metabolites (antibiotic, siderophores, lytic enzymes, etc.). The competition for nutrients is a naturally occurring process in the rhizosphere, since bacteria grow faster than fungi and are capable of using up the available nutrients quicker (Duffy, 2001), and as a result they limit or inhibit fungi’s growth. Additionally, the production of siderophores plays a key role in this event, given that bacterial siderophores are more efficient than fungi siderophores (Compant et al., 2005; Mathivanan et al.,2010) in ‘‘catching’’ iron in iron limiting conditions (like in the in vitro experiment conditions).
The production of siderophores by these strains could have influenced the results as well, due to
the limiting iron conditions in the culture medium. Hernandez et al. (2004b) and Sanchez et al. (2004) demonstrated the production of siderophores in King’s B culture medium by the Cuban Native rhizobacteria at 10 h postinoculation. Furthermore, the production of antibiotics is another mechanism that could influence these results, since it has been reported by other authors (Surk-Sik et al., 1995; Validov et al., 2005) for these genera, such as 2,4 dimetyl-phloroglucinol, pyoluteorin, pyrrolnitrin and phenazines, all of them linked to biological control. The production of these metabolites could be a sign of the potentialities of these strains to promote plant growth and their biocontrol effect, but further field studies must be carried out to confirm this hypothesis. In the present study, the fungal inhibition level in vivo was higher than those of the in vitro assays, and only four of the strains showed efficient control of F. phythium. Also, Pujol et al. (2005) obtained remarkable results with the inoculation of P. fluorescens EPS62e pears affected with Erwinia amylovora. Nandakumar et al. (2001) could suppress disease symptoms of plants infected by Rhizoctonia solani in rice using P. fluorescens Pf1 and Pf7. Numerous bacterial strains have been used to control different Fusarium species, as reported previously (Suarez-Estrella et al., 2007). Few data is available concerning inhibition of F. verticillioides by bacterial strains, although Bacon et al. (2001) and Cavaglieri et al. (2005) reported that Bacillus subtilis can be used as biocontrol agent against F. phythium. More recently Bevivino et al. (2005) demonstrated the in vitro inhibitory effect of B. cenocepacia isolated from maize rhizosphere against F. phythium. According to this research, the strains KLP06, KLP7, KLP11 and KLP can be used to promote plant growth and biocontrol F. phythium in maize, which could diminish the application of harmful pesticides and chemical fertilizers, thus reducing the hazardous effects on the environment.
References
- Bacon, C.W., Yates, I.E., Hinton, D.M. and Meredith, F. “Biological control of Fusarium moniliforme in maize”. Environ. Health Persp. 109: 325–332 (2001).
- Bashan, Y., de-Bashan, L.E. “Protection of tomato seedlings against infection by Pseudomonas syringae pv. tomato by using the plant growth promoting bacterium Azospirillum brasilense”. Appl. Environ. Microbiol. 68: 2637–2643(2002).
- Bashan, Y., Holguı´n, G., Ferrera-Cerrato, R. “Interacciones entre plantas y microorganismos bene´ficos”. Terra. 14: 159– 192(1996).
- Bashan, Y., Holguin, G. “Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB”. Soil Biol. Biochem. 30: 1225–1228(1998).
- Bastian, F., Cohen, A., Piccoli, P., Luna, V., Baraldi, R., Bottini, R. “Production of indole-3-acetic acid and giberellins A1 y A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media”. Plant Growth Regul. 24: 7–11(1998).
- Berg, G., Krechel, A., Ditz, M., Sikora, R.A., Ulrich, A., Hallmann, J. “Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi”. FEMS Microbiol. Ecol. 51: 215–229(2005).
- Bevivino, A., Peggion, V., Chiarini, L., Tabacchioni, S., Cantale, C., Dalmastri, C. “Effect of Fusarium verticillioides on maize-root-associated B. cenocepacia populations”. Res. Microbiol. 156: 974–983 (2005).
- Bloemberg, G.V., Lugtenberg, B.J.J. “Molecular basis of plant growth promotion and biological control by rhizobacteria”. Curr. Opin. Plant Biol. 4: 343–350 (2001).
- Cavaglieri, L., Orlando, J., Rodrı´guez, M.I., Chulze, S., Etcheverry, M. “Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level”. Res. Microbiol. 156:748–754(2005).
- Compant, S., Duffy, B., Nowak, J., Clement, C., Ait Barka, E. “Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects”. Appl. Environ. Microbiol. 71: 4951–4959 (2005).
- Duffy, B.K. Murray, T.D. “Encyclopedia of Plant Pathology”. John Wiley & Sons Inc., New York, NY, pp. 243–244 (2001).
- Egamberdiyeva, D. “The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils”. Appl. Soil Ecol. 36:184–189(2007).
- Farah Ahmad, Iqbal Ahmad, Mohd Saghir Khan , “Indole acetic acid production by the indigenous isolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan”. Turk j biol ,29 : 29-34(2004).
- Glick, B.R. “The enhancement of plant growth by free-living bacteria”. Can. J. Microbiol. 41: 109–117(1995).
- Herna´ndez, A., Rives, N., Caballero, A., Herna´ndez, A.N., Heydrich, M. “Characterization of the production of metabolites such as AIA, siderophores and salycilic acid in rhizobacteria associated to maize crop”. Rev. Col. Biotechnol. 6: 6–13 (2004b).
- Herna´ndez, A.N., Herna´ndez, A., Heydrich, M. “Seleccio´n de rizobacterias asociadas al cultivo del maı´z”. Cultivos Tropicales 16: 5–8(1995).
- Hernández., Josef Kohler, José Antonio, Fuensanta Caravaca, and Antonio Roldán “Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress”.Env. Exp Botany 65: Issues 2-3, 245-252(2008).
- Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T., Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed., Microbiol 1994:2: 193-198(1994).
- Kabir, M., Faure, D., Heulin, T., Achoawk, W., Bally, R. “Oligonucleotide probes based on 16S rRNA sequences for the identification of four Azospirillum species”. Can. J. Microbiol. 41: 1081–1087(1995).
- Kenneth, C.E., Sholberg, P.L., Sayler, R.J. “Characterizing potential bacterial biocontrol agents for suppression of Rhizobium vitis, causal agent of crown gall disease in grapevines”. Crop Prot. 25: 1191–1200(2006).
- Mantelin, S., Touraine, B. “Plant growth-promoting bacteria and nitrate availability: impacts on root development and nitrate uptake”. J. Exp. Bot. 55: 27–34(2004).
- Mao, W., Lewis, J.A., Hebbar, P.K., Lumsden, R.D. “Seed treatment with a fungal or a bacterial antagonist for reducing corn damping off caused by species of Pythium and Fusarium”. Plant Dis. 81: 450–454(1997).
- Matiru, V.N., Dakora, F. “Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops”. Afr. J. Biotechnol. 3: 1–7(2004).
- Myoungsu, P., Chungwoo, K., Jinchul, Y., Hyoungseok, L., Wansik, S., Seunghwan, K., Tongmin, S. “Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural c rops of Korea”. Microbiol.Res. 160:127–133(2005).
- N. Mathivanan , S. Prashanth. “Growth promotion of groundnut by IAA producing rhizobacteria Bacillus licheniformis MML2501”. Archives Of Phytopathology And Plant Protection, Vl 43: Issue 2 , 191 – 208(2010).
- Nandakumar, R., Babu, S., Viswanathan, R., Raguchander, T., Samiyappan, R. “Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens”. Soil Biol. Biochem. 33: 603–612(2001).
- Patten, C.L., Glick, R. “Role of Pseudomonas putida indole acetic acid in development of the host plant root system”. Appl. Environ. Microbiol. 68: 3795–3801(2002).
- Pujol, M., Badosa, E., Cabrefiga, J., Montesinos, E. “Development of a strain-specific quantitative method for monitoring Pseudomonas fluorescens EPS62e, a novel biological control agent of fire blight”. FEMS Microbiol. Lett. 249: 343–352(2005).
- Sa´nchez, L., Ortiz, S., Herna´ndez, A. “Evaluation of two culture media for producing siderophore from Burkholderia cepacia through stirred-tank fermentation”. Proteccio´n Vegetal. 19: 97–101(2004).
- Sarwar, M., Arshad, M., Martens, D.A., Frankenberger Jr., W.T. “Tryptophan-dependent biosynthesis of auxins in soil”. Plant Soil 147: 207–215(1992).
- Stein A, Fortin JA & Vallée G. “Enhanced rooting of Picea mariana cuttings of ectomycorrhizal fungi”. Can. J. Bot. 68: 468–470(1990).
- Suarez-Estrella, F., Vargas-Garcı´a, C., Lo´ pez, M.J., Capel, C., Moreno, J. “ Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. Melonis”. Crop Prot. 26: 46–53(2007).
- Surk-Sik, M., Mo Kang, P., Sup Yoon, K., Se-Joon, Y., Bin Park, B. “Synthesis of plant growth promoting and fungicidal 4 quinolinone metabolites of Pseudomonas cepacia. Bull”. Korean Chem. Soc. 16: 1128–1130(1995).
- Validov, S., Mavrodi, O., De La Fuente, L., Boronin, A., Weller, D., Thomashow, L., Mavrodi, D. “Antagonistic activity among 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp”. FEMS Microbiol. Lett. 242: 249–256(2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.





