How to Cite | Publication History | PlumX Article Matrix
Chemical Investigation of Annona Squamosa (Stem Bark)
Rakesh Ranjan1, Subra Singh1, Seema Tiwari2 and K. K. Singh2
Department of Medicinal Chemistry, Institute of Medical Sciences Banaras Hindu University, Varanasi - 221 005 India.
2Department of Chemistry, Udai Pratap P.G. College, Varanasi - 221 005 India.
ABSTRACT: 4, 9-Dihydroxy -3, 8-dimethoxy- benzo [4, 5] furo [3, 2-C] chromen -6- one, 6, 7'-dihydroxy -3- methoxydihydroflavonol, 5,7- dihydroxy -4'-methoxy isoflavone, 7-hydroxy -4'-methoxy isoflavone, 7,3'-dihydroxy -4'-methoxy isoflavone, 4', 5, 7-trihydroxy isoflavone, 2'- hydoxy genistein have been isolated for the first time from the stem bark of Annona squamosa and identified by spectroscopic data.
KEYWORDS: Annona squamosa; Spectroscopic data
Download this article as:| Copy the following to cite this article: Ranjan R, Singh S, Tiwari S, Singh K. K. Production of Alpha Amylase Using Bacillus Subtilis Ncim No. 2724 under Solid State Fermentation. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Ranjan R, Singh S, Tiwari S, Singh K. K. Production of Alpha Amylase Using Bacillus Subtilis Ncim No. 2724 under Solid State Fermentation. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9421 |
Introduction
Annonasquamosa(family Annonaceae) is native of Africa. It is also cultivated in India1. The decoction of the leaves of this plant is given for cholera2. In continuation to earlier work3 we investigated the stem bark of Annonasquamosaand isolated seven phenolic compounds for the first time from this source. Earlier workers isolated a wide variety of compounds viz. Amino acids4, terpenes5, sesquiterpenes6, diterpenes7, fats and oils8, steroids9, vitamins10, bezyltetrahydroisoquinaline11, proaporphines12, aporphines13, oxoaporphines14, and a large number of acetogenins15. The compounds are provisionally designated as AO-1 (I) to AO-7 (VII) and were characterised on the basis of their detailed spectroscopic analysis.
Isolation and Characterization of Compounds
The stem bark of Annonasquamosawere purchased from United Chemicals & Allied work, Clive Row-10, Kolkatta, India. The stem bark were milled by conventional method. Defatted milled stem bark of Annonasquamosa(5 kg) were extracted with methanol (15 l) for 16 hours. The methanolic extract (63.8 g) was then fractionated into four parts according to the increasing polarity of solvents viz, n-hexane chloroforms, ethylacetate and n-butanol. Chloroform soluble portion was chromatographed over SiO2 gel column and eluted with solvents of increasing polarity. The silica gel column chromatography of ethyl acetate soluble portion (see experimental) yielded seven compounds compund I to VII by elution with hexane-ethyl acetate 95 : 5 upto ethyl acetate by gradual increasing of the polarity of solvents.
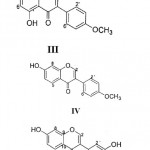 |
Scheme 1
|
AO-1 (I)
White crystals of AO-1 (I) MF C17H12O7 (M+ 328), m.p. 310°C responded positive to FeCl3 test. Its IR absorption bands showed the presence of unsaturated lactone (1716 & 1253 cm-1), hydroxyl group (3350 cm-1) and aromatic functionality (1608 and 1427 cm-1). The 1H NMR spectrum of AO-1 (I) showed the presence of one AB system [d 7.54 (1H, d, J=8.6 Hz, H-1)] and [d 7.00 (1H, d, J=8.6 Hz, H-2)] and one AX system [d 7.42, 1H, s and 7.20, 1H, s]. In addition to four proton signals the spectrum also showed two singlets (d 4.02 and 3.99, 3H, each) for two methoxyl group and two exchangeable signal at d 9.96 and 9.07 (1H, each) for two hydroxyl group.
The ¹³C NMR, nOe studies in 1H NMR and UV spectral studies bathochromic shift with (AlCl3 and H3BO4) has indicated the structure of AO-1 (I) as 4, 9-dihydroxy – 3, 8 – dimethoxy – benzo [4, 5] furo [3, 2-C] chromen – 6 – one16.
AO-2 (II)
Greenish crystals, m.p. 210-211°C. IR absorption bands showed hydroxyl group (3333 cm–1) aromaic ring (1515 and 1741 cm–1) and a carbonyl group (1653 cm–1) in its molecule, UV absorption bands at lmax 216, 235 276 and 341 nm and bathochromic shift of band II with MeOH + NaOAc suggested the presence of 7-OH group, addition of boric acid also showed bathochoromic shift of band II, thus confirming o-dihydroxyl group. 1H NMR spectrum has suggsted ring B as unsubstituted [d7.55 (2H, m, H-2′ & H-6′) and (d 7.45, 3H, m, H-3′, H-4′ and H-5′)] while two singlets at d 7.19 and 6.43, 1H each was assigned to H-5 and H-8. The only methoxy singlet at d 3.80 was assigned to methoxyl at C-3 position. Other two nonaromatic hydrogens at d 5.14 and 4.53, dd where assigned to H-2 and H-3 respectively. Thus compound was identified as 6, 7- dihydroxy -3- methoxydihydroflavonol.
AO-3 (III)
C16H12O5 (MH+ at 285), white needles from methanol, m.p. 215-216°C. The UV data and the UV with shift reagents suggested it to be 5, 7- dihydroxy flavonoid. Its isoflavone nature was deduced by characteristic signal for H-2 at d 8.19 (1H, s) and C-2 at d 15.5 ppm. The ¹H NMR data AX system (d 6.28 and 6.42, ¹H d, each J= 1.9 Hz) and A2B2 system (d 7.5 and 6.98 2H each d, J= 8.8 Hz) along with two exchangeable hydrogens and a methoxy singlet (d 3.91) has allowed us to assign the structure of AO-3 (III) as 5, 7- dihydroxy -4′- methoxyisoflavone commonly known as biochanin17.
AO-4 (IV)
C16H12O4 (M+ 268) white crystal, m.p. 258°C showed in its IR spectrum absorption bands at 3424 cm–1 (hydroxyl group) and 1624 cm–1 (conjugated carbonyl group). The isoflavone nature of AO-4 (IV) was established by 1H NMR signal at d 8.17 (1H, s). The 1H NMR spectrum also showed one ABX system in A ring [ d8.07 (1H, d, J=9.2 Hz) d 6.99 (1H, dd, J = 9.2 and 2.1 Hz and d 6.90 (1H, d, J=2.1 Hz)] and an A2B2 system in B ring [d 7.56 (2H, d, J=8.7 Hz) and d 6.97 (2H, d, J=8.7 Hz)]. The position of hydroxyl and methoxyl groups were established by study of shift reagents in UV spectrum. On the basis of these data and its comparison with reported literature data18, 19 AO-4 (IV) was identified 7- hydroxy -4′- methoxyisoflavone commonly known as formonentin.
AO-5 (V)
C16H12O5 (MH+ 285), colourless needles from methanol, m.p. 241-245°C. IR spectrum showed absorption bands for hydroxyl (3420 cm–1) carbonyl (1624 cm–1) and aromatic (1580 cm–1) functionalities. The UV spectrum in methanol showed lmax 224, 247 and 288 nm while in NaOAc band II showed a bathochromic shift which confirmed the presence of hydroxyl group at C-7 position in flavonoid.
¹H NMR spectrum of AO-5 (V) confirms the isoflavone nature (d 8.83, 1H s, d c 153.2 s). The 1H NMR spectrum also showed two ABX system in the molecule one in ring A [d 8.03 (1H, d, J = 8.7 Hz) d 7.02-6.97 (1H, m) and d 6.92 (1H, d, J=2 Hz)] for H-5, H-6 and H-8 respectively and the other ABX system of B ring at d 7.09 1H and 7.02, 6.92, 2H, m). The spectrum also showed the presence of a methoxy singlet at d 3.84. The compound was established as 7, 3′ – dihydroxy -4′- methoxyisoflavone by nOe studies, the irradiation of methoxy signal at d 3.84 enhanced the signal for H-5′. This compound AO-5 (V) was thus identified as calicosin by comparison of the spectral data reported in literature20.
AO-6 (VI)
C15H10O5 (MH+ at m/z 271), yellow needles m.p. 301-302°C. IR showed absorption bands for hydroxyl (3430 cm–1) and carbonyl (1650 cm–1) functionalities. UV absorption maximum in methanol was found at 262 and 337 nm. Use of shift reagents has suggested the presence of phenolic group at 5 and 7 position. The presence of isoflavonic 2-H was settled by 1H NMR signal (d 8.31, 1H, s) and A2B2 system in B ring (d 7.22 and 6.81, 2H, d, each, J=8.6 Hz) and AX system in A ring (d7.13 and 6.21, 1H, d each J=1.8 Hz) Thus settled the structure of AO-6 (VI) as 4′, 5, 7 trihydroxyisoflavone a commonly known isoflavone by comparison with reported data21,22.
AO-7 (VII)
C15H10O6 (MH+ at M/z 287), m.p. 270-273°C. The IR absorption bands at umax 3350 and 1655 cm–1 and UV absorption maxima (lmax 258 and 315 nm) taken in consideration with molecular formula has suggested it to 2′, 4′, 5, 7- tetrahydroxyisoflavone. This is found to be in full agreement with 1H NMR data which showed AX system in A ring and ABX system in B ring along with characteristic 2-H. The compound was readily recognised as 2′-hydroxy genistein, by comparison with reported spectroscopic data in literature²³.
Experimental
The melting point were measured on a Yazawa hot stage microstage apparatus and are uncorrected. Optical rotations were measured on JASCO DIP-360 Polarimeter (cell length 5 cm). UV absorption spectra were recorded on JASCO UV/visible spectrophotometer (model no. 7800) while IR on JASCO FT-IR 5300 spectrometer. 1H and 13C NMR data of compounds I, II, III, IV, V, VI, VII recorded in CDCl3 at 300 MHz and 75 MHz respectively.
Compound I
White light crystals, m.p. 310°C, EI-MS : m/z 328 [M]+, IR (KBr) umax cm-1 3350, 2928, 1716, 1601, 1520, 1427, 1253 and 745, UV lmax nm (MeOH) 208, 244, 345; (MeOH + NaOAc) 210, 245, 347; (MeOH + NaOAc/ H3BO3) 208, 244, 347, (MeOH + NaOMe) 209, 245, 378, (MeOH + AlCl3) 209, 247, 345, (MeOH + AlCl3/ HCl) 208, 244, 344
¹H NMR : d 9.96 (1H, s, 9-OH); 9.07 (1H, s, 4-OH); 7.54 (1H, d, J=8.6 Hz, C-1H); 7.42 (1H, s, 4-OH) 7.20 (1H, s, C-10-H); 7.00 (1H, br, d, J=8.6 Hz, C-2-H); 4.02 (3H, s, 3-OCH3); 3.99 (3H, s, 8-OH-3)
15C NMR:d 161.0 (C-6); 159.0 (C-3); 157.0 (C-10a); 154.6 (C-11a); 149.8 (C-4); 147.2 (C-8); 147.1 (C-9); 135.4 (C-4a); 116.6 (C-1); 114.4 (C-6b); 114.1 (C-2); 105.5 (C-6a); 102.6 (C-11b); 102.2 (C-7); 99.4 (C-10); 61.0 (3-OCH3); 56.4 (8-OCH3)
Compound II
Greenish crystals, m.p 210-211°C, [a]22D + 13.3 (methanol + CHCl3, c, 0.06), FAB-MS m/z 287, IR (KBr) vmax 3333, 1653, 11515, 1471, 830 and 766 cm–1, UV lmaxnm (MeOH) 341, 276, 235, 216; (MeOH + NaOAc) 346, 255, 218; (MeOH + AlCl3) 377, 238, 218, (MeOH + AlCl3 + HCl) 339, 236, 219
1H NMR : d 7.55 (m, C-H -2′) 7.55 (m, C-H-6′); 7.48-7.41 (m, C-H-3′); 7.48-7.41 (m, C-H-4′); 7.48-7.41 (m, C-H-5′); 7.19 (1H, s, C-H-5); 6.43 (1H, s, C-H-8) 5.68 (1H, br d, 1 × OH); 5.14 (1H, d, J=11.6 Hz, C-H-2); 4.53 (1H, dd, J=3.7, 11.6 Hz, C-H-3); 3.80 (3H, s, 3-OCH3)
¹³C NMR : d 192.5 (C-4); 157.5 (C-9); 155.3 (C-7); 144.3 (C-1′); 137.9 (C-6) 128.8 (C-2′); 128.5 (C-3′); 128.5 (C-5′); 128.5 (C-6′); 128.3 (C-4′); 110.0 (C-10); 107.6 (C-8); 103.6 (C-5); 84.0 (C-3); 72.9 (C-2); 56.2 (3-OCH3)
Compound III
White needles, m.p 215-216°C, FAB-MS m/z 285 [M+ H], 569 [2M+ H], IR (KBr) vmax 3500, 1724, 1687, 1606, 1594, 1455, 1383, 1246, 1181, 1043 cm–1, UV lmaxnmMeOH : 261, 330 sh; MeOH + NaOAc : 272, 327; MeOH + AlCl3 + HCl) 273, 310 sh, 373
¹H NMR : d12.85 (1H, s, 5-OH); 8.19 (1H, s, C-H-2); 7.54 (2H, d, J=8.8 Hz, C-H-2′) 7.54 (2H, d, J=8.8 Hz, C-H-8′); 6.98 (2H, d, J=8.7 Hz C-H-3′); 6.98 (2H, d, J=8.7 Hz, C-H-5′); 6.42 (1H, d, J=2.1, C-H-8); 3.91 (3H, s, 4-OCH3)
¹³C NMR : d180.5 (C-4); 165.1 (C-7); 163.9 (C-5); 160.7 (C-4′); 159.1 (C-9); 154.5 (C-2); 131.1 (C-6′); 124.1 (C-3); 123.7 (C-1′); 114.4 (C-3′); 114.4 (C-5′); 106.1 (C-10); 99.1 (C-6); 94.4 (C-8); 56.3 (4-OCH3)
Compound IV
White crystals, m.p. 240°C, EI-MS m/z 284 [M]+, IR (KBr) vmax 3500, 1724, 1687, 1606, 1594, 1455, 1383, 1246, 1181, 1043 cm–1, UV lmaxnmMeOH : 261, 330 sh; MeOH + NaOAc : 272, 327; MeOH + AlCl3 + HCl : 273, 310 sh, 373
¹H NMR : d 9.72 (1H, s, 7-OH); 8.17 (1H, s, H-2, C-H-2); 8.07 (1H, d, J=8.7 Hz, C-H-5); 7.56 (2H, d, J=8.7 Hz, C-H-2′) 7.56 (2H, d, J=8.7 Hz, C-H-6′); 6.99 (1H, dd, J=9.2 Hz, 2.1 Hz, C-H-6); 6.97 (2 H, d, J=8.7 Hz, C-H-3′); 6.97 (2H, d, J=8.7 Hz, C-H-5′) 3.83 (3H, s, 4′-OCH3)
13C NMR : d 162.7 (C-7); 159.1 (C-4′); 157.6 (C-9); 152.6 (C-2); 147.8 (C-4); 130.3 (C-6′); 130.0 (C-2′); 127.2 (C-5); 124.4 (C-1′); 123.5 (C-3); 116.8 (C-10); 115.1 (C-6); 113.5 (C-3′); 113.5 (C-5′); 102.1 (C-8); 56 (4′ – OCH3)
Compound V
Colourless crystals, m.p. 245-247°C, FAB-MS m/z 285 [M+H]+, IR (KBr) lmax 3420, 1624, 1580, 1510, 1470, 1381, 1023, 853 cm–1, UV lmaxnm MeOH : 288, 247, 224; MeOH + NaOAc : 327, 255, 221; NaOAc + boric acid : 288, 247, 225
¹H NMR : d 9.10 (1H, br. hump, 4′ OH) 8.33 1H, s, C-H-2); 8.03 (1H, d, J=8.7 Hz, C-H-5);7.09 (1H, C-H-2′); 7.02-6.97 (C-H-6); 7.02-6.97 (C-H-5′); 7.02-6.97 (C-H-6′) 6.92 (1H, d, J=2.0 Hz, C-H-8); 3.84 (3 H, s, 7- OCH3)
¹³C NMR : d 177.8 (C-4) d 162.6 (C-4′); 157.5 (C-3′); 153.2 (C-2); 147.6 (C-7); 146.1 (C-10); 127.4 (C-5); 124.7 (C-9); 123.5 (C-1′) 119.8 (C-2′); 116.7 (C-4); 116.5 (C-5′) 115.3 (C-6); 112.0 (C-6′); 55.7 (7-OCH3)
Compound VI
Bright yellow needles, m.p. 301-302°C, FAB-MS m/z 271 [M+H]+, IR (KBr) vmax 3430, 2920, 1650, 1617, 1571, 1510, 1465, 1240, 1188, 1170 cm–1, UV lmaxnmMeOH : 337, 262
1H NMR : d 12.94 (1H, s, 5-OH) d 9.56 (1H, br. hump, 7- OH); 8.31 (1H, s, C-H-2); 7.72 (2H, d, J=8.4 Hz, C-H-2′); 7.72 (2H, d, J=8.4 Hz, C-H-2′) 7.72 (2H, d, J=8.4 Hz, C-H-6′) 7.13 (1H, d, J=1.8 Hz, C-H-6); 6.81 (2H, d, J=8.4 Hz, C-H-3′); 6.81 (2H, d, J=8.4 Hz, C-H-5′); 6.21 (1H, d, J = 1.8, C-H-8)
13C NMR : d 181.3 (C-4); 164.7 (C-7); 163.7 (C-5); 158.8 (C-9); 158.2 (C-4′); 154.0 (C-2); 130.9 (C-2′); 130.9 (C-6′);123.8 (C-3); 122.9 (C-1′); 115.8 (C-3′); 115.8 (C-5′); 106.0 (C-10); 99.7 (C-6); 94.3 (C-8)
Compound VII
White needles, m.p. 270-273°C, FAB-MS m/z 287 [M+H]+, IR (KBr) umax 3350, 1655, 1575, 1500, 1464, 1234, 1178, 1104 cm–1, UV lmax nm MeOH : 315 (sh), 258, MeOH + AlCl3 : 315 (sh), 268; MeOH + AlCl3 + HCl : 315 (sh), 268
¹H NMR : d 12.97 (1H, s, 5-OH); ´ 9.29 (2H, br. d, 2 × OH) 8.13 (1H, s, C-H-2); 6.95 (1H, d, J=8.4 Hz, C-H-6′); 6.36 (1H, d, J=1.5, C-H-6); 6.34 (1H, d, J=2.1, C-H-3′); 6.25 (1H, d, J=8.4, 2.1 Hz, C-H-5′); 6.20 (1H, d, J=1.5 Hz, C-H-8)
¹³C NMR : d 180.5 (C-4); 164.2 (C-5); 161.2 (C-7); 158.6 (C-4′); 157.7 (C-9); 156.5 (C-2′); 155.3 (C-2); 132.2 (C-6′); 120.5 (C-3); 108.7 (C-1′); 106.3 (C-5′); 104.5 (C-10) ; 102.7 (C-3′); 98.9 (C-6) 93.7 (C-8)
References
- The Wealth of India : Raw Materials, A Dictionary of Indian Raw Materials and Industrial products, Council of Scientific and Industrial Research, New Delhi, 1: 80-81(1948).
- Glossary of India Medicinal Plants – CSIR New Delhi, India, p. 25 (1956).
- Rupprecht, J.K., Hui, Y.H. and McLaughlin, J.L., J. Nat, Prod., 53: 237 (1990).
- Touche, A., Desconclois, J.F., Jacquemin, H., Lelievre, Y. and Forgacs, P. Plant. Med. Phytother. 15: 4 (1981).
- Luciana Alves, Rodrigues Dos Santos, Maria Amelia, DiamatinoBoaventura, Lucia Pinheiro, Santos Pimenta, Biochemical Syntematics and Ecology 34: 78-82 (2006).
- Xiao-Xi Liu, Elsa Pilorinon and Jerry L McLaughlin Tetrahedron Letters 40: 399-402 (1999).
- AlassaneWele, Celine Landon, Henri Labbe Francoise Vovelle, Yanjun Zhang and Bernard Bodo, Tetrahedron 6: 405-414 (2004).
- Kawashima, A., Kishimoti, M. Morimoti, S., Akyama, T., Maejima, A., Kawada, I., Japanese Patent JPO841068, 1996, Chem. Abstr. 124: P298921C (1996).
- Ishikawa, T., Sekine, K. Japanese Patent JPO1122871, 2001, Chem. Abstr., 134: P344563f (2001).
- Lion Corp. Japanese Patent JP 2000191542-A, 10 (2000)..
- Xu, S., Zhang, G., Song, S., Meng, S., Cao, Y, Xu, B., Lu, J. Cinese Patent CN 1370537, 2002, Chem. Abstr. 139: P255374F (2003).
- Sassa, S., Sakamoto, S., Zhou, YF., Mori, T., Kikuchi, T., Shinoda, H. In-Vivo 15: 25 (2001).
- Xie, H., Wu, T., Huang, L., Li, C. ZhongguoZhong Yao ZaZhi22: 238 (1997).
- Rao, S.V. Ramachandran, K. and Zaher, S.H., J.I.C.S. Ind. and News Ed. 18: 215 (1955).
- Ghanekar, RRV and Ayyar, P.R. J. Indian Inst. Sci., 10A: 28 (1927)
- Farnsworth, N.R. Blomster, R.N. Quimby, M.W. and Schermerhorn, J.W., The Lynn Index Monograph, 3: 60 (1974).
- Oliveros – Belardo, L., Lloydia, 38: 537 (1075).
- Rao, R.V.K. Murty, N., Rao, J.V.L.N. and Seshagiri, Indian J. Pharm. Sci. 40: 170 (1978).
- Bohlmann, F. and Rao, N., Chem. Ber., 106: 841 (1973).
- Hernandez. M., Agronomia(Cuba), 3: 58 (1943).
- Yang, T.-H. and Chan, C-M. J. Cem. Soc. (Taipei), 17: 243 (1970).
- Balbaa, S.L. Haggag, M.Y. and Taha, K.F. Egypt. J. Pharm. Sci. 18 : 1 (1979).
- Jolad, S.D. Hoffmann, J.J., Schram, K.H., Cole, J.R., Tempesta, M.S., Kriek, G. R. and Bates, R.B. J. Org. Chem., 47: 3151 (1982).
- Lieb. F., Nonfon, M., Wachendorff – Neuman, V. and Wendisch D., Planta Med. 56: 317 (1990).
- Kawazu, K., Alcantero, J.P. and Kobayashi, A., Agric. Biol. Chem., 53: 2719 (1989).

This work is licensed under a Creative Commons Attribution 4.0 International License.





