How to Cite | Publication History | PlumX Article Matrix
Comparative Study of Various Entraped Techniques of Immobilization Using Amylase
M. P. Kusuma, J. Archana and B. Archana
B. V. R. R. Women’s College of Pharmacy, Bharkatpura, Hyderabad - 27 India.
ABSTRACT: In this study, alpha-amylase was immobilized in various matrices like sodium alginate, polyacrylamide, agar agar and gelatin. The present objective is to compare the activity of the immobilized enzyme by various methods in hydrolyzing the starch molecule. The activity of the enzyme immobilized by sodium alginate was found to be higher than the remaining methods.
KEYWORDS: Amylase; immobilization; sodium alginate; polyarylamide; gelatin
Download this article as:| Copy the following to cite this article: Kusuma M. P, Archana J, Archana B. Comparative Study of Various Entraped Techniques of Immobilization Using Amylase. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Kusuma M. P, Archana J, Archana B. Comparative Study of Various Entraped Techniques of Immobilization Using Amylase. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9683 |
Introduction
Enzymes are biological catalysts. They increase the rate of chemical reactions taking place within living cells without themselves suffering any overall change. Amylases are among the most important industrial enzymes and are of great significance in present day biotechnology. Two major classes of amylases have been identified namely α-amylases and glucoamylases in addition to this β-amylases, which is generally plant origin has also been reported a few microbial sources. Amylases are characterized by their ability to hydrolyze glucosidic linkages in polysaccharide. Alpha-amylases (endo 1, 4-α–D–glucan glucohydrolase, EC 3.2.1.1) are extracellular and acts on starch components. The α-amylases may be derived from several bacteria, yeasts and fungi. Bacterial amylase, however, is generally preferred over fungal amylase due to its thermostability and also it minimizes the contamination risk and reduces reaction time. Strains of Bacillus sp., mainly Bacillus subtills, Bacillus licheniformis, Bacillus amyloliquefaciens and Bacillus megaterium are employed. Among these Bacillus subtilis strains are employed for commercial application1,3 .
Physical entrapment of enzymes in calcium alginate beads has shown to be a relatively easy, rapid and safe technique. Entrapment requires that the immobilized enzyme should be a large enough molecule to be kept inside the gel matrix but the substrate and product should be small enough to pass through the pores of the gel 2.
Starch molecules are very large, often reaching a molecular weight of 80 million Daltons. It is expected that starch hydrolysis reaction could occur more effective if enzyme bound to surface. Therefore, a comparison of different methods of immobilization of α -amylase to calcium alginate method would provide useful information on the efficiency of the hydrolysis of starch into smaller sugars.
In this study amylase which was purchased commercially from Himedia was immobilized and compared with that of the immobilized enzyme synthesized by the bacteria and comparative study of different immobilization methods was drawn.
Materials and Methods
Immobilization by Sodium alginate entrapment method
Sodium alginate purchased from Himedia was prepared by dissolving 3gm sodium alginate in 100ml of boiling water and autoclaved at 121°C for 15minutes.Both alginate slurry and enzyme equivalent to 0.03gm were mixed and stirred for 10 minutes to get a uniform mixture. The slurry was taken into a sterile syringe and added drop wise into 0.2M CaCl2 solution from 5cm height and kept for curing at 4° C for 1 hour The cured beads were washed with sterile distilled water 3 to 4 times. When the beads were not being used, they were preserved in 0.9% sodium chloride solution in the refrigerator. All operations were carried out aseptically under laminar flow unit 4,5.
Immobilization of Amylase in Agar-agar
A definite quantity of agar-agar (Hi media, Mumbai, India) was dissolved in 18ml of 0.9% sodium chloride solution to get a final concentration of 2% and sterilized by autoclaving . The enzyme suspension (2ml equivalent to 0.03gm) was added to the molten agar-agar maintained at 40° C shaken well for few seconds (without forming foam ), poured into a sterile flat bottom 4 inch diameter petriplates and allowed to solidify. The solidified agar block was cut into equal size cubes(4 mm3) added to sterile 0.1M phosphate buffer (pH 7.0 ) and kept in the refrigerator for curing. After curing phosphate buffer was decanted and the cubes were washed with sterile distilled water 3to 4 times 6.
Immobilization of the enzyme in Gelatin
5ml of 0.06% of enzyme suspension was added to 15ml of 20% sterile gelatin (Hi media) maintained at 45° C and poured into a sterile petridish. The gel was over layered with 10ml of 5% glutaraldehyde for hardening at 30°C .The resulting block was cut into small size cubes and the cubes were washed thoroughly with sterile distilled water for the complete removal of excess glutaraldehyde 6.
Immobilization of the enzyme in Polyacrylamide
A cell suspension was prepared by adding 0.03g cells to 10ml chilled sterile distilled water. To another 10ml of 0.2M sterile phosphate buffer(pH 7.0), the following chemicals were added: 2.85g acrylamide,0.15g bisacrylamide ,10mg ammonium persulphate and 1ml TEMED (NNN1 N1tetra methyl ethylene diamine). The cell suspension and the above phosphate buffer mixture well and poured into sterile flat bottom 10cm- diameter petriplates. After polymerization the acryl amide gel was cut into equal size cubes(4mm3) transferred to 0.2M phosphate buffer(pH 7.0) and kept in the refrigerater for 1 hour for curing. The cubes were washed 3 to 4 times with sterile distilled wate and stored in sterile distilled water at 4 C until use 7.
Production of amylase
Bacillus subtilis was isolated from the soil and identified by Berger’s manual of systematic bacteriology. The organism was grown in 250ml of Erlenmeyer’s flask, each containing 50ml of starch nutrient broth medium that has the following composition(g/L) : beef extract ,3.0.,peptone 10.0.,NaCl ,5.0., Starch,10.0., distilled water 1000ml. The pH was adjusted to 7.0 . The flasks were inoculated with spore suspension obtained from nutrient agar and incubated at 40°C with shaking at 150rev/min at 48hr. The filtrates was then separated by centrifugation at 10,000/10min.The reaction mixture was estimated by taking 0.4ml of 1%soluble starch and 0.5ml of each diluted culture supernatant and 1% phosphate buffer . The mixture was incubated at 50°C for 20min and incubated at 10,000rpm and assayed for reducing sugar8 .
Assay of amylase activity
Amylases acts on starch to produce reducing sugars which upon reaction with DNS reagent form orange to reddish colored complex having maximum absorbance at 540nm. To the above immobilized enzyme 1ml of 1% starch solution and 1ml of distilled water was added and incubated. After incubation 1ml of 2N NaOH to stop the enzyme reaction . 1ml of DNS reagent was added and placed in the boiling water bath for 10min and OD was measured at 540nm against blank in colorimeter. 1 units of amylase activity is expressed as the amount of enzyme which produces 1m mole per min of reducing sugar with glucose as standard.
Standard graph of maltose was drawn and with the help of standard graph, enzyme activity was calculated. The activity of enzyme immobilized by various methods was calculated and further compared with the activity of the enzyme produced by Bacillus subtilis.
Results and Discussion
Among the immobilization methods activity of enzyme entrapped by sodium alginate was more than that of remaining entrapment methods(2.4 IU/ml). Sodium alginate method is having an advantage that it is non-ionic. The consequence is that the properties of the enzymes are only minimally modified in the presence of the gel matrix. At the same time, the diffusion of the charged substrate and products is not affected, gelation of calcium alginate does not depend on the formation of more permanent covalent bonds between polymer chains. Rather, polymer molecules are cross-linked by calcium ions.
Immobilization in gelatin is having a next higher value after sodium alginate entrapment(1.6 IU/ml) . The main attraction of using gelatin as the immobilization media is that the gel formation process requires only simple equipment and that the reagents are relatively inexpensive and nontoxic. The retention of enzymatic activities for immobilization with a gelatin gel is typically 25-50% of the original free enzyme. Gelatin gel has the advantage that the mass transfer resistance is relatively low compared to other entrapment methods, but the rate of enzyme loss due to leakage is high.
The activity of the enzyme immobilized in polyacrylamide and agar agar were found to be less than remaining methods.Among these two methods polyacrylamide gave high titer value than agar agar. The results are tabulated in Table.1.
Table 1: Standard graph of Maltose
| Vol of std maltose(ml) | Vol. of DNS(ml) | Conc. Of Maltose(mg) | OD at 540nm
|
| 0 | 1 | 0 | 0 |
| 0.2 | 1 | 0.4 | 0.02 |
| 0.4 | 1 | 0.8 | 0.04 |
| 0.6 | 1 | 1.2 | 0.06 |
| 0.8 | 1 | 1.6 | 0.03 |
| 1 | 1 | 2 | 0.1 |
Table 2: Activity of Amylase immobilized by different methods.
| Method | Activity
( U/ml )
|
| Entrapment by Sodium alginate | 2.4 |
| Entrapment by Gelatin | 1.6 |
| Entrapment by Polyacrylamide | 1.2 |
| Entrapment by agar agar | 0.1 |
| Free Enzyme | 3.8 |
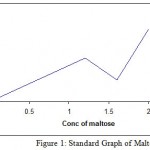 |
Figure 1: Standard Graph of Maltose.
|
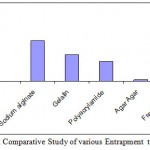 |
Figure 2: Comparative Study of various Entrapment techniques.
|
Unlike the adsorption and covalent bonding methods, most polymerization reactions that cause cross-linking and gel formation in entrapment methods do not directly involve the formation of bonds between the support material and the enzyme molecules. There are reports that these bonds change the conformation of the enzyme protein and modify the enzyme properties. Since the enzyme molecules do not themselves participate in the polymerization reaction in the entrapment methods, the same entrapment techniques can be successfully applied to a wide range of enzymes with only minor modifications between different enzymes.
References
- Mohamed A., Abdel-Naby and Amal M. Hashem ., MicrobioL Res., 153, 1-000 (1998).
- Emne’us J. and Gorden L.Anal.Chim.Acta., 234,97-106(1990).
- Mifflin T.E. and Bruns D.E., Clin Chem , 615-620 (1987).
- Roy I., Sastry, M. S. R., Johri, B. N. and Gupta, M. N., Enzyme Microb. Tehnol.,27,53-65(2000).
- Johnsen A.and Flink JM.,Enzy.Microb. Technol.,8,737-748(1986).
- Veelken M., Pape H., J.Appl Microbiol Biotechnol., 15,206-210(1982).
- rabhakaran G,Pugulvendhan R.,1(4),189-194(2009).
- Bajpai P and P.Bajpai .,Biotechnol.Bioengg.,33,72-78 (1989).

This work is licensed under a Creative Commons Attribution 4.0 International License.





