How to Cite | Publication History | PlumX Article Matrix
P. Sannasi1*, S. Salmijah2 and J. Kader2
Faculty of Applied Science, INTI International University, Persiaran Perdana BBN, Putra Nilai, 71800 Nilai, N. Sembilan Malaysia.
2Faculty of Science and Technology, Universiti Kebangsaan Malaysia- 43600 UKM Bangi, Selangor D.E. Malaysia.
Corresponding Author E-mail:palsan.abdullah@newinti.edu.my
ABSTRACT: This paper reports the capability of consortium culture (CC) comprising of an acclimatized mixed bacterial culture to withstand the toxic effect of Cr(VI), Cu, and Pb, at 1, 10, 100 mg/l and its uptake, and to remove heavy metals from an industrial effluent. Consortium culture displayed good heavy metal resistance (75-84.6%) on nutrient agar. Inverse of heavy metal toxicity index, B (l/mg) reflected CC’s ability to tolerate Cr(VI) concentration of up to 507.6 mg/l, followed by Pb at 348.43 mg/l and Cu at 243.90 mg/l. High metal uptake capacity was observed at 1 mg/l (q = 4.47-10.33 mg/g), 10 mg/l (q = 29.27-96.07 mg/g) and 100 mg/ (q = 85.28-175.02 mg/g) in nutrient broth. Overall, metal toxicity was in the order Cu > Pb > Cr(VI), and metal uptake was Pb > Cu > Cr(VI). X-ray fluorescence screening indicated the abundance of Ca, K, P, and S on the biomass. Heavy metal removal study demonstrated that CC was able to grow in waste effluent which was not subjected to any pre-treatment or nutrient addition. Significantly higher metal removal in the range of 92-97.5% (P < 0.05) was obtained for Cd, Cr(VI), Cu, Ni, and Pb with CC. Furthermore, CC was able to thrive and compete in the presence of indigenous microbial population with no apparent decrease in metal removal capability. In conclusion, results establish the feasibility of employing CC to remove heavy metals from industrial effluents and support the development of a bacterium-based integrated waste treatment system.
KEYWORDS: Consortium culture; metal removal; metal resistant; metal uptake; industrial effluent
| Copy the following to cite this article: Sannasi P, Salmijah S, and Kader J. Effect of Heavy Metals to Bacterial Culture and the Removal of Heavy Metals from an Industrial Effluent. Biosci Biotechnol Res Asia 2010;7(2) |
| Copy the following to cite this URL: Sannasi P, Salmijah S, and Kader J. Effect of Heavy Metals to Bacterial Culture and the Removal of Heavy Metals from an Industrial Effluent. Biosci Biotechnol Res Asia 2010;7(2). Available from: https://www.biotech-asia.org/?p=8814 |
Introduction
Heavy metal pollution in the environment is of great concern to scientists as well as environmentalist as metallic ions are known to be recalcitrant in nature and non-biodegradable as opposed to many other xenobiotic compounds. The phenomenon of environmental pollution is a global issue nowadays. Heavy metals generally refer to metals exhibiting > 5 g/cm3 in atomic density and usually known for its toxicity and associated with pollution (Nies, 1999). Its presence in potable waters is potentially hazardous to health. The main generator of heavy metal containing waste are the metal finishing and plating, metallurgical works, film processing, electrical and semiconductors, agriculture (fertilizer, pesticide, fungicide), petroleum and gas operations, and from the combustion of fossil fuels (Doble and Kumar, 2005). In Malaysia, the implementation of strong industrial plans towards achieving vision 2020 to become a developed nation has seen the establishment of various industrial estates to drive the manufacturing sector. However, the same emphasis cannot be said in aspects relating to industrial waste and effluent treatment, and management. Major water pollution point sources are mainly from the sewage treatment plants (48.3%) and manufacturing industry (45.1%) (Malaysia Environmental Quality Report 2007, 2008). Monitoring of the environmental regulation compliance by local enforcement agencies showed that industries like metal finishing and electroplating, metal industries, metal fabrication, chemical printing, pharmaceutical, plastics, electric and electronics, and water treatment plants are among industries that could only achieve not more than 79% compliance to Environmental Quality (Sewage and Industrial Effluents) Regulations, 1979 (Department of Environment, 2007) hence becomes the main source of pollution compared to other industries. Some premises even operate without effluent treatment plants while some plants had treatment protocols that are not capable of treating the effluent to meet the allowable limits. The marine water quality monitoring in Malaysian waters revealed the increasing trend in samples polluted with heavy metals (i.e. copper, lead, cadmium, and chromium) from the years 2005 to 2007 (Malaysia Environmental Quality Report 2007, 2008). Several other studies conducted in the coastal waters of the South China Sea particularly in Malaysia, Thailand, Philippines, Vietnam, and Indonesia over the past two decades also point to similar findings (United Nations Environment Programme, 2007). This is noteworthy as marine water quality reflects the degree of pollution from land based sources as well as those from the sea.
The hazardous effect of heavy metals towards nature and man is commonly known and has been widely reported [Nies, 1999; Department of Environment, 2007; United Nations Environment Programme, 2007). In addition, the untreated wastewater discharged by these industries is often contaminated by a multitude of other harmful substances as well. These substances can damage the sewers and interfere with the treatment methods at water treatment facilities (Doble and Kumar, 2005). This is more pronounced in developing countries where manufacturing industries have become ever so important to lead the economy. Among the challenges faced by the industry these days is to comply with environmental regulations that requires heavy metal containing wastewater to be treated prior to discharge (Department of Environment, 2007). Treatments subjected to the industrial waste effluent if any, involve a combination of conventional physico-chemical methods which are very costly, inconsistent in performance, and have many technical limitations. Among others the need for high reagent use, being waste specific, not consistent in the presence of mixed waste, inefficient at low metal concentrations, and the generation of toxic chemical sludge will always be of concern (Eccles, 1999).
This creates much interest towards alternative treatment techniques such as those employing microorganisms, bacteria in particular. The use of microbial cells to facilitate bioremediation of metal contaminated environment presents an alternative solution. Bacterial cell are known to carry genetic information governing detoxification and/or resistance capabilities. A variety of resistance and/or detoxifying mechanisms which may be of intrinsic or extrinsic property in nature have been documented in microorganisms exposed to heavy metals [Nies, 1999; Cheung and Gu, 2007). Further to this, the bacterial trait to remove heavy metals can generally be referred to as bioaccumulation and biosorption, and can be carried out by both active and inactive cells (Sannasi et al., 2006). Thus the understanding of bacteria-metal interactions will always be beneficial as pollution conditions can vary from one site to another. Although many studies have successfully reported the isolation of metal resistant bacterial cells and subsequently followed by metal uptake studies in the laboratory, very little research has been carried out to explore and measure the suitability of these isolates to work with real wastes and real conditions in the field. The effectiveness of a biological metal removal treatment system is not only dependent on biomass characteristics but also upon the biological, physical and chemical nature of the effluent. In addition to the heavy metal ions present, industrial effluents especially those from metal-based industries are known to contain many anionic element, cyanide complexes, borates, organic acids and substances, catalyst, solvents, chelating agents, as well as oil and grease (Doble and Kumar, 2005; El-Bestawy, 2008). The presence of these compounds can affect metal removal capability and may even inhibit growth of laboratory grown bacterial cells. As such, a simulation study needs to be carried out to evaluate the real potential of any treatment system of interest.
On this note, the effect of heavy metals towards growth and metal uptake, and the ability of CC to remove metals from an industrial effluent were thus investigated to determine the efficiency, effectiveness, and practicality of this consortium culture for future bioremediation application in the field. This will be useful to impart further information and data in the development of a bacterium-based biosorbent for treating industrial waste and wastewater.
Materials and Methods
Source of bacterial culture and growth conditions
This study was carried out by using a well characterized bacterial mixed culture, collectively known as consortium culture (CC) (Sannasi et al., 2006; Sannasi et al., 2009). The mixed culture was sourced from a pool of bacterial isolates originating from point and non-point sites of areas related to metal-based activities (Sannasi et al., 2000). Consortium culture (CC) was maintained in a basal medium containing yeast extract (0.5 g/l), peptone (0.5 g/l), and NaCl (8.5 g/l) as either a growth culture or an acclimatized culture, with fortnightly media refreshments. The acclimatized culture was supplemented initially with 1 mg/l of each Cr(VI), Cu(II), and Pb(II); and the concentration was subsequently increased to 10 mg/l each. The growth culture on the other hand was void of heavy metals for maximal biomass production. The bacterial cultures were grown at room temperature (28-30°C) at an initial pH of 6.8 ± 0.2. Bacterial growth was monitored by optical density (OD) at 600 nm (spectrophotometer; Hitachi U1100, Japan); plate counts as colony forming units/ml (cfu/ml) and correlated dry weight (g) of biomass.
Starter cell inoculum preparation
To prepare starter cell inoculum, aliquots (0.5% v/v) from growth and metal acclimatized cultures were each inoculated into 10 ml basal media (as described above) supplemented with Cd(II), Cr(VI), Cu(II), Ni(II) and Pb(II), at 0.2 mg/l each resulting in the total metal concentrations of 1 mg/l. Initial pH was set at 6.8 ± 0.2 and incubated under static conditions at room temperature for 48 h. Cell biomass was separated by centrifugation (4000 r/min, 10 min); pellets were washed, re-centrifuged and rinsed twice before re-suspended in saline. An inoculum size of 1% (v/v) standardized to cell density at OD600 of 0.500 (containing approx. 107 cells/ml).
Heavy metal resistance, bacterial growth and metal uptake study
Investigation into CC’s resistance to heavy metals was performed on both solid (with nutrient agar, NA) and liquid (nutrient broth, NB) media spiked with 1, 10 and 100 mg/l of Cr(VI), Cu and Pb each. For test utilizing NA, 100 μl of starter inoculum (as described above) was spread on NA plates and incubated at room temperature for 48 h. Bacterial growth was recorded as mean log cfu/ml. Percentage of resistance (R, %) was determined by Eq. (1):
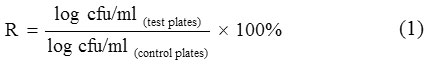
For test in NB, 1% (v/v) starter inocula was inoculated into NB (total volume was made to 10 ml) and incubated under static conditions at room temperature for 48 h. Initial pH was set at 6.8 ± 0.2. Bacterial growth was followed by optical (OD) at 600 nm (spectrophotometer; Hitachi U1100, Japan) and compared to control. The dose-response effect is expressed by Eq. (2):

where, y is the level of growth (OD600) of cells at metal concentration of C (mg/l); a, is the level of growth (OD600) in control (in the absence of metal) culture and, metal toxicity index, B (l/mg). The inverse of B (1/B, mg/l) will serve as an indicator of the heavy metal concentration limit the bacterial population can withstand (Malakul et al., 1998). Following which, bacterial biomass was harvested by centrifugation (4000 r/min, 10 min); pellets were washed and re-centrifuged. This step was repeated twice to clean the cell biomass pellet from media residues as well as non-sorbed metals. Cell pellet was then prepared for metal uptake analyses as described in the section below. Metal uptake (q) was determined by the Eq. (3) (Sannasi et al., 2006; Kratochvil and Volesky, 1998):

where, q is the metal uptake (mg metal/g dry weight biomass) in solution, V (ml). Cp is the amount of metal contained (mg/l) in the biomass of known dry weight, w (g).
Heavy metal analyses in biomass
Harvested bacterial biomass from above was digested with concentrated HNO3 at 60-65°C for 15-16 h prior to total metal determination (Sannasi et al., 2006). Concentrations of Cr(VI) in the media was determined spectrophotometrically using the 1,5-diphenylcarbazide method (American Public Health Association, 1992; Kader et al., 2007) and total metal concentrations in the media and the cells was measured by atomic absorption spectrophotometer (AAS; Perkin Elmer 1100B, USA). Test values obtained were deducted by values measured in control sets to substantiate for abiotic metal losses. All values are mean of triplicates.
Major elements screening by x-ray fluorescence (XRF) spectrophotometer
The non-destructive qualitative XRF technique was used to screen and identify the presence of major elements i.e. Ca, K, P, and S, and to ascertain the effect of heavy metals exposure towards their presence. One ml of starter inoculum was suspended in ddH2O containing a mixture of Cd, Cr(VI), Cu, Ni and Pb at 20 mg/l each for 24 h (total volume was 10 ml). The suspension was centrifuged (4000 r/min, 10 min) and the supernatant discarded. The pellet was suspended in 10 ml of ddH2O and re-centrifuged. This step was repeated 3 to 5 times to remove any media residues and unsorbed metals from the solution and biomass. Pooled biomass was then suspended in 1-2 ml of ddH2O before frozen at -80°C and subjected to lyophilisation (Labconco, USA). Sample in powder form (~ 0.5 g) was prepared as pressed-pellet with 6 g of boric acid (BH3O3) as the matrix for analysis. Three sets were prepared; (i) negative control (boric acid matrix only), (ii) positive control (metal free biomass), and (iii) test (metal exposed biomass). The XRF (x-ray fluorescence spectrophotometer; Philips PW1480, USA) was performed by using methane/argon gas mixture in the flow proportional counter equipped with rhodium (Rh) x-ray tube. Intensity was measured as kilocounts per second (kc/s) and peak transition monitored at 2q°. Other analysis parameters are presented in Table 1.
Table 1: Analysis parameters for qualitative screening of major elements by x-ray fluorescence spectrophotometer (XRF).
| Elements | Analysis crystal type | Detection angle (2q°) |
| Ca | LiF 200 | 113.01 |
| K | LiF 200 | 118.07 |
| P | GE | 141.03 |
| S | GE | 110.62 |
Scanning angle: 105°-145°; operated at 40 kV and 60 mA
Scanning time: 30 min
Study area
The area of interest was the nearby Bukit Serdang Industrial Estate located in Seri Kembangan, Selangor. The heavy metal removal simulation was carried out by using an industrial effluent sample collected from one of the drainage that flows through a chromium plating premise, two lots of metal works establishment, two automotive workshops, a tannery, and an office appliance operator. The specified drainage runs into a main storm water sewer system.
Sampling
Sampling was performed according to standard water and wastewater examinations protocol (American Public Health Association, 1992). Based on the focus of the study, the effluent sample was characterized of its properties, i.e. heavy metal content, pH, and bacterial population. Samples were sorted into two parts; one for the determination of bacterial count, and the other for heavy metal analyses. Concentrations of Cd, Cu, Ni, and Pb in the sample were determined by atomic absorption spectrophotometer (AAS; Perkin Elmer 1100B, USA). Concentration of Cr(VI) was determined by the 1,5-diphenylcarbazide complexation method described previously (American Public Health Association, 1992; Kader et al., 2007).
Bacterial growth and heavy metal removal from effluent sample
Four sets of test (A-D) were initiated for the simulation, and their respective conditions are shown in Table 2. The purpose was to simulate and compare the bacterial growth and metal removal efficiency of the bacterial populations. The effluent samples in Set A and B is not autoclaved.
Table 2: Test conditions for metal removal simulation in effluent sample.
| Test sets | Prior sterilization | Description |
| A | No | Indigenous bacterial population |
| B | No | Indigenous bacterial population + consortium culture (CC) |
| C | Yes | Consortium culture (CC) |
| D | Yes | Control |
In order to initiate the test, 1.5 ml aliquots of CC inocula suspended in saline was added into the effluent samples (Set B and Set C) with no nutrient addition. Initial pH was noted and no adjustment was made as to mimic natural conditions in the field. The final volume was made to 150 ml. Culture bottles were shaken at 150 r/min (orbital shaker, Yihder TS-580, Taiwan) (Ganguli and Tripathi, 1999) at room temperature. Experiments were done in triplicates as batch cultures and mean values are reported.
For analyses purposes, 15 ml aliquots were drawn out daily for 5 days; 10 ml of which was centrifuged and the pellet suspended in 10 ml of ddH2O and re-centrifuged. This step was repeated twice to clean the biomass pellet from effluent residues as well as non-sorbed metals. Biomass pellet was then prepared for metal analyses as above (Sannasi et al., 2006). Percentage (%) metal removal was calculated by Eq. (4):
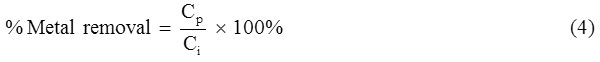
where, Cp is the amount of metal contained (mg/l) in the biomass and, Ci is the initial concentration of metals in the effluent (mg/l).
The remaining 5 ml aliquot was used for bacterial growth determination. Bacterial growth was monitored by optical density (OD) at 600 nm (spectrophotometer; Hitachi U1100, Japan) and plate counts, and reported as log cfu/ml.
Heavy metals stock solution
Metals solutions were prepared from the following salts with NANOpure ddH2O; Cd(II) from Cd(NO3)2.4H2O, Cr(VI) from K2CrO4, Cu(II) from Cu(NO3)2 2.5H2O, Ni(II) from Ni(NO3)2.6H2O and Pb(II) from Pb(NO3)2. Test solutions were prepared by diluting stock solutions (1000 mg/l) to the desired concentrations in ddH2O. Metal concentrations were determined by atomic absorption spectrophotometer (AAS; Perkin Elmer 1100B, USA).
Statistical Analysis
Experimental data were subjected to statistical analysis for mean tests, t-tests, Levene tests, least squares regression and the analysis of variance (one-way ANOVA) by SPSS (SPSS Inc., Chicago, USA). Significant levels were set at a = 0.05.
Results
Heavy metal resistance, bacterial growth and metal uptake
High percentage of resistance, R (75-84%) was observed with all tested metals at all concentrations on NA (Table 3). Cells were more resistant and grew better in the presence of Pb at all concentrations except at 1 mg/l but the difference between metals and concentration was insignificant (P < 0.05). This indicated that all the metals (Cr(VI), Cu, and Pb) at 1-100 mg/l were not toxic to cell growth. In our observations, dark brown colored and layered colonies were pertinent on agar containing metal ions suggesting active accumulation of metal from the agar. The color became more intense after 48 h to 72 h indicating progressive metal immobilization and accumulation. However, no attempt was made to quantify this.
Table 3 also shows the extent of metal toxicity, metal tolerance limit and metal uptake in NB. Copper was the most toxic metal seen from the higher B values; 0.134 l/mg, 0.0391 l/mg, and 0.0041 l/mg at 1, 10 and 100 mg/l metal concentrations, respectively (P < 0.05). The least toxic metal was found to be Cr(VI) at 1 mg/l (B = 0.0241 l/mg), 10 mg/l (B = 0.0198 l/mg) and 100 mg/l (B = 0.00197 l/mg) (P < 0.05). These values are inversed to reflect the highest theoretical concentration of metal ions that can be tolerated by CC. Among the tested metal ions, CC have better tolerance to Cr(VI) of up to 507.6 mg/l, followed by Pb at 348.43 mg/l and Cu at 243.90 mg/l. The highest metal uptake was observed with Pb at all concentrations tested (P < 0.05), i.e. at 1 mg/l (q = 10.33 mg/g), 10 mg/l (q = 96.07 mg/g), and 100 mg/l (q = 175.02 mg/g). Overall, toxicity was in the order of Cu > Pb > Cr(VI), and metal uptake trend was Pb > Cu > Cr(VI).
Table 3: Data on percentage resistance (%), metal toxicity index (B, l/mg), metal tolerance limit (inverse of B, mg/l) and metal uptake (q, mg/g) of Cr(VI), Cu, and Pb at 1, 10, and 100 mg/l.
| Metals | Cr(VI) | Cu | Pb | ||||||
| mg/l | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 |
| R | 84.6 | 75.6 | 76.9 | 84.6 | 78.2 | 76.9 | 80.8 | 80.8 | 84.6 |
| B | 0.0241 | 0.0198 | 0.00197 | 0.134* | 0.0391* | 0.0041* | 0.0926 | 0.0213 | 0.00287 |
| 1/B | 41.49 | 50.50 | 507.60 | 7.46 | 25.58 | 243.90 | 10.79 | 46.95 | 348.43 |
| q | 4.47 | 29.27 | 85.28 | 5.28 | 37.46 | 115.01 | 10.33* | 96.07* | 175.02* |
R: percentage (%) of resistance
B: metal toxicity index (l/mg)
1/B: metal tolerance limit (mg/l)
q: metal uptake (mg/g)
*: value significant at P < 0.05.
Major elements screening by x-ray fluorescence (XRF) spectrophotometer
Figure 1 and 2 depicts the differences in specific peak detection for the tested element (Ca, K, P, and S) between control and test sets. Negative control (boric acid matrix) did not show any peak detection for the tested elements. Positive control (biomass not exposed to metals) and test sample (biomass exposed to metals) showed the presence of Ca, K, P and S with specific peaks detected at 113.01 2q° (for Ca), 118.1 2q° (for K) (Fig. 1), 141.03 2q° (for P) and 110.62 2q° (for S) (Fig. 2). The signal intensity for K, P, and S increased while Ca signal intensity decreased in biomass exposed to metals (test) as opposed to control biomass.
 |
Figure 1: X-ray fluorescence (XRF) spectrum for the screening of P and S. A- negative control (boric acid matrix); B- positive control (biomass not exposed to metals); C- test sample (biomass exposed to Cd, Cr(VI), Cu, Ni, and Pb. Specific peak detection at 141.03 2qo (for P) and at 110.62 2qo (for S) confirming the presence of P and S. |
 |
Figure 2: X-ray fluorescence (XRF) spectrum for the screening of Ca and K. A- positive control (biomass not exposed to metals); B- test sample (biomass exposed to Cd, Cr(VI), Cu, Ni, and Pb. Specific peak detection at 113.01 2qo (for Ca) and at 118.1 2qo (for K) confirming the presence of Ca and K. |
Effluent sample characterization
Table 4 shows that the pH value of the effluent sample is in the range of 6.1-6.2, with temperature of 29-30oC. The heavy metal concentration varied between the 5 metals (Cd, Cr(VI), Cu, Ni and Pb) tested and fell in the range of 0.20 mg/l (Cd) to 3.83 mg/l for Cu. The order is as follows (in mg/l): Cu > Pb > Cr(VI) > Ni > Cd. Nevertheless, the presence of all metals exceeded the allowable limit set by the local environmental regulations (Environmental Quality Act and Regulations handbook, 1996). Indigenous bacterial count in the effluent sample was quite low in the range of 3.6 × 104 – 2.3 × 105 cfu/ml. No attempt was made to characterize the indigenous bacterial population.
Table 4: Characterization of effluent sample (pH values, temperature, indigenous bacterial count, heavy metals content).
| Parameters | Effluent sample characterization | |||||
| pH | 6.1-6.2 | |||||
| Temperature (°C) | 29-30°C | |||||
| Indigenous bacterial population (cfu/ml) | 3.6 × 104 – 2.3 × 105 | |||||
| Heavy metals | Cd(II) | Cr(VI) | Cu(II) | Ni(II) | Pb(II) | |
| Initial metal concentration (mg/l) | 0.20 | 1.57±0.03 | 3.83±0.02 | 0.52±0.02 | 3.78±0.07 | |
| Permissible limit (mg/l) a | 0.01;0.02 | 0.05;0.05 | 0.20;1.00 | 0.20;1.00 | 0.10;0.50 | |
a: Limit allowed for industrial effluent under Standard A (above the water intake) or Standard B (below the water intake) under the Third Schedule, Environmental Quality (Sewage and Industrial Effluents) Regulations 1979), Environmental Quality Act 1974 (Environmental Quality Act and Regulations Handbook, 1996).
Bacterial population dynamics
Figure 3 illustrates the bacterial population count in the respective test sets for the duration of 5 days. In general, optimum bacterial growth in all sets was attained after 24 h. Growth in sets containing CC (Sets B and C) was significantly higher compared to indigenous population (P < 0.05). The highest growth was recorded with set C (solely CC) on day 2 at 3.09 × 108 cfu/ml; this was followed by growth in set B (1.19 × 108 cfu/ml on day 2) and set A (2.56 × 106 cfu/ml on day 2). This was followed by sustained growth until day 3, as bacterial growth began to drop the next day onwards which may reflect the depletion of nutrient and energy source and/or of metal toxicity. Subsequent spread plate and streak plate confirmed that CC’s component isolates were more dominant compared to the indigenous bacterial isolates from the field (plates not shown). This was evident from the abundance of Pseudomonas sp and Serratia sp, which is exclusive to CC (Sannasi et al., 2006; Sannasi et al., 2009). The ability of CC to proliferate and sustain growth with no external nutrient addition indicated the presence of substrates that can be utilized as carbon and energy source in the effluent sample.
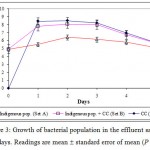 |
Figure 3: Percentage (%) removal of metal from the effluent sample over 5 days. Ci: Initial metal concentration. Readings are ± standard error of mean (P < 0.05). |
The removal of Cd, Cr(VI), Cu, Ni, and Pb from effluent sample
The capability of both indigenous bacterial population and CC was evaluated based on maximum or final percentage (%) removal attained, and highest overall removal % mean (calculated over the 5 days). Figure 4 depicts the % removal of the tested metals from the effluent sample. Table 5 summarizes % metal removal to the residual metal concentration (Cr) measured on day 5.
Copper: Figure 4 shows that the highest removal of Cu of up to 92.2% was observed with CC on day 5. The metal concentration dropped from Ci = 3.83 mg/l to Cr = 0.30 mg/l (Table 5). The highest overall removal % mean was also observed with CC (65.6%), followed by CC + indigenous population (50.6%), and indigenous population (9.5%). Abiotic loss was 2.4%. The highest overall removal % mean was significantly different (P < 0.05) between all populations except between CC and CC + indigenous population.
Lead: Consortium culture (CC) showed the best metal removal capability among all the sets with the highest overall removal % mean of 76% (P < 0.05) compared to CC + indigenous population (51.1%), indigenous population (15.2%) and control (3.5%). The level of Pb in the sample with CC was lowered the most by 97.5% (Fig. 4) to Cr of 0.09 mg/l (Table 5) on day 5.
Chromium: The highest overall metal removal % mean was observed with CC (72.8%) as opposed to CC + indigenous population (44.6%), indigenous population (15.2%), and control (5.7%) (P < 0.05). Figure 4 shows that highest metal removal was attained by CC (95.7%) with a Cr reading of 0.07 mg/l (Table 5) on day 5 (P < 0.05) followed by CC + indigenous population (63.7%) and the lowest removal with indigenous population (15.2%). Abiotic loss was recorded at 8.9%.
Nickel: Maximal removal percentage of up to 96.2% (P < 0.05) was observed with CC on the 5th day (Cr = 0.02 mg/l) (Fig. 4; Table 5). Highest overall metal removal % mean was read at 71.5% with CC, followed by CC + indigenous population (35.4%), and with indigenous population (25%). Highest overall metal removal % mean was significantly different between CC and indigenous population (P < 0.05). Abiotic loss of Cd was recorded at 7.8% (control; set A).
Cadmium: The highest overall metal removal % mean was recorded with CC (84%) and was significantly different (P < 0.05) between CC + indigenous population (63.3%), indigenous population (37%), and control (8%). Maximum % removal was achieved with CC (95%) on day 5 (Fig. 4) with residual metal concentration level of 0.01 mg/l (Table 5). Maximum removal % however, was not significantly different (P > 0.05) between CC and CC + indigenous population (80%). However, no significant difference was noted between CC and CC + indigenous population.
During the 5 days period of incubation, the abiotic metal removal (control set) was insignificant when compared to the test sets (P > 0.05) and was in the range of 2.9-15%. The better metal removal efficiency was observed with CC, and CC + indigenous population as opposed to indigenous population alone. The presence of CC elevated metal removal efficiency of all metals (P < 0.05). Maximum metal removal % of Cd, Cr(VI), Cu, Ni and Pb in all sets for the period of 5 days is presented in Table 5. The metal removal % by indigenous population is in the range 13.9-45%, far lower with what is achieved by CC. Consortium culture (CC) + indigenous population was able to remove 48.1-80% and the highest % metal removal was achieved by CC of up to 92.2-97.5%. Removal tendency was in the order: Cd > Pb > Cr(VI) > Ni > Cu. The final residual metal concentration (Cr) in CC sets was well below the permissible level of local regulations (Environmental Quality Act and Regulations Handbook, 1996) (Table 4 and Table 5). The results gained in this study affirm the ability of CC to strive and remove heavy metals field effluent sample.
 |
Figure 4: Percentage (%) removal of metal from the effluent sample over 5 days. Ci: Initial metal concentration. Readings are ± standard error of mean (P < 0.05). |
Table 5: Percentage (%) removal of metal from the effluent sample by bacterial populations over 5 days.
| Bacterial cells | % Metal removal | ||||
| Cd(II) | Cr(VI) | Cu(II) | Ni(II) | Pb(II) | |
| Control | 15.0 ± 2.9 | 8.9 ± 2.0 | 2.9 ± 0.4 | 5.8 ± 2.9 | 5.6 ± 0.8 |
| Indigenous population | 45 ± 0.3 | 17.2 ± 4.0 | 13.9 ± 3.0 | 34.6 ± 1.4 | 21.4 ± 2.0 |
| CC + indigenous population | 80.0 ± 2.9 | 63.7 ± 2.2 | 73.0 ± 0.6 | 48.1 ± 5.9 | 71.7 ± 1.5 |
| Consortium culture (CC) | 95 ± 0.1 | 95.7 ± 2.2 | 92.2 ± 1.5 | 96.2 ± 2.9 | 97.5 ± 0.1 |
| Cr (mg/l)a | (0.01 ± 0.003) | (0.07 ± 0.03) | (0.3 ± 0.06) | (0.02 ± 0.01) | (0.09 ± 0.01) |
a: Residual metal concentration (Cr) level after 5 days in samples containing consortium culture (CC).
Discussion
Bacterial growth on agar gave a qualitative indication on the ability of the bacterial population to heavy metals. The observation of dark colored layered colonies on agar strongly suggested the ability of CC to accumulate and immobilize heavy metal ions from the media. Similar observations of brown colourations and darkening in the presence of the metal ions tested had been reported (Hernandez et al., 1998). This proved the ability of CC to thrive in metal containing media and at the same time immobilize metal ions. Overall, CC displayed good resistance towards Cu, Cr(VI) and Pb (75-84.6%) on NA. A Trichococcus sp isolate was reported to show resistance percentage of 25% (with Pb) and 47% (with Cu) at 100 mg/l, and 45.8% with Cr at 125 mg/l in yeast-peptone media (Basu et al., 1997). Although no further attempt was made to quantify metal accumulation in this part, the subsequent study in broth culture was performed to address this. Cells were grown with metals in NB to observe the relation between metal toxicity (B) and metal uptake (q). Higher B value indicates greater metal toxicity effect. A more resistant population would give a lower B (l/mg) reading. The inverse value of B indicated the highest threshold limit for metal tolerance (mg/l). It was determined that CC was more tolerant to Cr(VI) (507.6 mg/l), and Cu was the most toxic metal. In general, metal uptake followed the trend Pb > Cu > Cr(VI) at all tested concentrations. However, no correlation was observed between metal toxicity, B and metal uptake, q (P < 0.05). Although Cr(VI) was least toxic, metal uptake was in the lower range (4.47-85.28 mg/g) as opposed to Cu (5.28-115.01 mg/g) and Pb (10.33-175.02 mg/g). This can be explained due to the differing cell response that can be observed towards Cr(VI). Many mechanisms of metal resistance have been presented amongst them are, intra- and extracellular metal sequestration, metal reduction, metal efflux pumps, and the production of metal binders such as metallothioneins and biosurfactants (Ehrlich, 1997; Nies, 1999) which can dictate metal uptake. One of the reported cell defense mechanism towards heavy metals is the ability to reduce toxic metals to a less toxic valence form (Cheung and Gu, 2007; Zahoor and Rehman, 2009); in this case CC is likely to have reduced Cr(VI) to Cr(III). Chromium(III) is less toxic to the cell, and it precipitates in the media thus explaining lower metal uptake albeit better cell resistance and tolerance. A higher tolerance to Cr(VI) of up to 2500 mg/l with reducing capability at concentrations lower than 1500 mg/l had been discussed previously (Camargo et al., 2003) with a Bacillus sp. High tolerance (up to 4800 mg/l) and reducing ability (up to 85%) towards Cr(VI), and up to 800 mg/l with Pb(II), and up to 200 mg/l with Cu(II) by a Bacillus sp. JDM-2-1 had also been noted (Zahoor and Rehman, 2009). However in both these studies, metal uptake capability was not tested and a single culture was used for a single metal. The natural micro biota is characterized by the presence of a mixed variety of microbial population, and exposed to a multitude of pollutants at any time as represented in this study with CC. On the whole, CC demonstrated good growth and resistance to metal toxicity amid high metal uptake capability with Cr(VI), Cu and Pb at concentrations of 1, 10, and 100 mg/l. Competition and acclimatization within the CC members may have produced strains that are more resilient in the presence of metallic ions. This shows the advantages of employing resistant and non-resistant strains together as sometimes metal tolerant strains protect the other strains in the environment by forming insoluble metal complexes in the form of metal sulfides, metal phosphates, metal carbonates, among others, thus minimizing the toxic effect to the whole bacterial population. At the same time, metal uptake and immobilization can occur which is beneficial for the whole remediation process. Spectroscopic study revealed the abundance of Ca, K, P and S in CC which can naturally act as metal chelating ligands as well as participate in ion exchange. The intensity of S, P and K was shown to be elevated whereas the intensity of Ca diminished in cells exposed to metals in contrast to control biomass. The reduction in Ca signal with biomass exposed to metals suggests the occurrence of divalent (i.e. Cd, Cu, Ni, and Pb) ion exchange resulting in Ca to be released into the solution. Similar reduction in Ca intensity after Cu binding was observed in moss cells (Asheh and Duvnjak, 1997). The slight increase in K may be due to K2CrO4 substance used in this study. However, further quantitative analysis is needed to elucidate the overall process for better understanding. The energy dispersive x-ray (EDX) coupled with x-ray diffraction (XRD) analyses will be carried out in the future to address this query.
Some researchers have noted that metal ions are precipitated on the cell surface in the initial stage to prevent them from penetrating the cell membrane and causing cell death due to metal toxicity (Kratochvil and Volesky, 1998; Cheung and Gu, 2007). However, at the same time some metal ions do pass through to the cytoplasm actively by the aid of specific and non-specific transport systems. In addition, metal ions can also enter passively due to puncture or permeability changes experienced by the cell membrane (Nies, 1999). Sensitive cell will die but certain cells will develop resistance and become adapted to heavy metal and may result in the occurrence of mutant cells. In the cell interior, the metal ions can undergo sequestration in specific organelles or bound to metal chelating molecules. In addition, intracellular metal concentrations can be regulated by diverse transport systems which pump out (efflux) metal ions (Ehrlich, 1997). These metal efflux homeostasis system can form part of the cell’s defense and detoxification mechanism. However, this will result in lower metal uptake as observed with Cr(VI) in our study. Nevertheless there are studies (Nies, 1999; Sar et al., 2001; Zahoor and Rehman, 2009) which have demonstrated that the ions pumped out are precipitated as metal hydroxides, phosphates or carbonates around the cell’s periplasmic space and on cell wall. This in turn will act as nucleation sites for immobilizing and precipitating more metal ions from the solution passively. Again this depends on the availability of suitable chemical moiety on cell surface such as S and P. In our study, CC has been shown to harbor many of the elements (Ca, K, P, and S) which can enhance metal complexation as well as ion-exchange. And the lessen detection of these elements after exposure to metal ions strongly suggests its role in increasing overall metal uptake capability. Investigation of metal binding by CC under transmission electron microscopy (TEM) recently (Sannasi et al., 2009) have proven the occurrence of metal deposits extracellularly due to the production of exopolysaccharides, and intracellularly as a result of active and passive metal uptake. Understandably, a multitude of metal removal and metal response mechanisms maybe working simultaneously to ensure the survival of the population, what more in a mixed bacterial culture such as CC. In order to transform this positive outcome to a workable solution, a metal removal simulated study with industrial effluent was carried out to evaluate practicality of CC for field application.
The choice of metals analyzed (i.e. Cd, Cr(VI), Cu, Ni, and Pb) were the common heavy metal pollutant found at increased level in the locality as determined from our earlier study (Sannasi et al., 2000) and also emphasized due to their toxicity [Nies, 1999; Dhamotharan et al., 2008]. As seen from Table 4, it is clear that the waste effluent must undergo an appropriate treatment in order to remove the heavy metals present to levels that are acceptable under the local environmental regulations before released into the water bodies. As the level of heavy metals is not extremely high (0.20-3.83 mg/l), any physico-chemical approach will be impractical and economically non-feasible. This situation can be best addressed by a bacterium-based system such as CC which can work in a broad range of heavy metal concentrations. Another advantage of employing a bacterium-based system is the possibility to treat co-existing wastes. Industrial waste effluent are known to harbor other hazardous wastes which needs to be treated as well, which can be done simultaneously thus being more cost-effective. Interestingly, CC also has the potential to thrive in various hydrocarbon containing wastes (unpublished findings) and is being examined further. The bacterial culture can actually utilize any nutrient and organic/carbon sources present as their energy source whilst removing the metallic ions.
When grown in the effluent sample, CC was observed to be significantly more resilient and better as opposed to the growth of indigenous bacterial population. From the point of bacterial growth, the reduced bacterial population observed after day 3 (Fig. 3) may reflect the depletion of nutrient and carbon source and/or of metal toxicity. The former limitation could be overcome by employing larger starter inoculum size and/or introducing nutrient supplement, depending upon the volume of the effluent to be treated. Other researchers (Ganguli and Tripathi, 1999) demonstrated that the growth and reduction of chromate in an effluent sample originating from a tannery by P. aeruginosa was attenuated by the addition of a carbon source, nitrogen and phosphorous. In another study (Jacques et al., 2008) elevated degradation of polycyclic aromatic hydrocarbons (PAHs) of up to 90% was demonstrated when the microbial consortium was inoculated together with nutrients. Although nutrient addition can be advantageous for microbial growth (Vidali et al., 2001); however the effect towards indigenous and introduced bacterial population will have to be probed further. The question, whether the nutrient will be directly beneficial to the working culture is difficult to determine over time as it will be hard for any strain to attain dominance solely.
For the latter, it can be presumed that growth was inhibited due to metal toxicity. Nonetheless, our previous investigations have ascertained that CC in its inactive form (dead biomass) can still function as an excellent heavy metal biosorbent (Sannasi et al., 2006; Kader et al., 2007). Overall, metal removal % of up to 92-97.5% observed with CC in this study is comparable if not better to the other systems reported in the literature. A cyanobacteria (T. ceytonica) was used to remove Cu (94.63%) and Zn (86.12%) from a domestic-industrial wastewater (El-Bestawy, 2008). On the other hand a Bacillus sp JDM-2-1 was shown to reduce Cr(VI) up to 86% in industrial effluents over a period of 6 days (Zahoor and Rehman, 2009).
Ideally it is accepted that waste minimization in industrial processes is the first and foremost important step to reduce waste generation than the treatment options that follows later. However, a large portion of untreated industrial waste effluent will find its way into the aquatic environment. Unfortunately, these wastes from the industry, in particular heavy metals are hazardous and will pollute the water bodies. These persistent substances will then interfere with water treatment methods at water treatment facilities. This will increase the cost of operation with the need for damage repairs and sludge treatment and disposal. The use of microbial biomass in the biosorption of heavy metals is an essential part in the integrated approach of wastewater treatment. The unwanted effects can be eliminated with the introduction of a cost effective, dynamic and flexible pre-treatment approach of industrial effluent by the use of CC in simple reactors or for in situ conditions. A real-time experimental design will need to be in place to gauge implementation practicality.
Implementation of bioremediation is on its own a dynamic process. Prediction of the process itself can be risky and results are best determined empirically by monitoring of the process in the field. This includes monitoring the hydrological, chemical and biological conditions over the life span of a project. The nature of many industries greatly varies in terms of size, processed raw materials, and products formed. More than often, the waste generated during industrial production processes is of vast diversity, such as their chemical composition, temperature, and volume (Doble and Kumar, 2005). Some points to consider would be as to whether it is better to introduce an encapsulated inoculum, or the need to look into possible seasonal variation of the microbial community, and changes to non-controllable environmental factors i.e. availability of contaminants, type of soil, or type of effluent, temperature, pH, the presence of oxygen or other electron acceptors and nutrients (Vidali, 2001). The latest technological advances in bioaugmentation and biostimulation research consist of genetic fingerprinting and molecular markers to determine the interactions between augmented organisms and native organisms (pre-existing biomass) will be very helpful in understanding the microbial interactions better. In addition, there are some prospects for genetically engineered microorganisms in this area. Genetic improvement can aid in developing existing technologies to cater for waste decontamination. This approach pose a greater advantage of not only being cost-effective and dynamic, but can be effectively upgraded and combined with other new emerging techniques to further enhance and improve the success of industrial effluent bioremediation. For example, one can combine with the action of cyanobacteria which was shown to remove Cr effectively from tannery effluent waste (Dhamotharan et al., 2008). Nonetheless, more study especially those done directly on site have to be carried out before any approach or process is applied as to evaluate it s economical and technical feasibility. The combinatory features of metal resistance with good metal uptake in bacterial cells as seen with CC is highly sought to address problems in heavy metal waste bioremediation. Results showed that CC displayed higher percentage of tolerance and better metal uptake of the selected metal ions. The outcome of our findings during the 5 days period clearly established that the growth of CC and the efficiency of metal removal were not deterred by the conditions of waste effluent from the field even with no external nutrient addition. The extent of metal removal by CC was at its best with removal of up to 92-97.5% from the initial metal concentrations of the mixed metals (Cd, Cr(VI), Cu, Ni, and Pb) found in the effluent sample. This coupled to the documented high metal loading capability of CC makes it a promising candidate in the development of a bacterium-based biosorbent for local field bioremediation in time to come. Future studies will look into needs to immobilize the consortium culture (CC) on a suitable matrix for the development of a bacterium-based biosorbent which not only caters for heavy metals but for the complex mixture of industrial waste, waste effluent and wastewater as well.
Acknowledgements
This study was funded by the Ministry of Science, Technology and Innovation, Malaysia (No. 06-01-02-SF0469).
References
- Nies D.H., Microbial heavy metal resistance. Appl. Microbiol. Biotechnol., 51:730-750 (1999).
- Doble, M. and Kumar, A., Treatment of waste from metal processing and electrochemical industries. In: Biotreatment of Industrial Effluents. Burlington MA: Elsevier Butterworth-Heinemann, 145-155 (2005).
- Malaysia Environmental Quality Report 2007, Department of Environment, Ministry of Natural Resources and Environment, Kuala Lumpur, Malaysia, 56-59, 62 (2008).
- Department of Environment, Annual Report 2007, Department of Environment, Ministry of Natural Resources and Environment, Kuala Lumpur, Malaysia, 60-61. (2007).
- United Nations Environment Programme, Land-based pollution in the South China Sea, UNEP/GEF/SCS Technical Publication No. 10. Bangkok, Thailand (2007).
- Eccles, H., Treatment of metal-contaminated wastes: why select a biological process? Trends Biotechnol., 17: 462-465 (1999).
- Cheung, K.H. and Gu, J., Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int. Biodeterior. Biodegrad., 59(1): 8-15 (2007).
- Sannasi, P., Kader, J., Ismail, B.S. and Salmijah, S., Sorption of Cr(VI), Cu(II) and Pb(II) by growing and non-growing cells of a bacterial consortium. Bioresour. Technol., 97: 740-747 (2006).
- El-Bestawy, E., Treatment of mixed domestic-industrial wastewater using cyanobacteria. J. Ind. Microbiol. Biotechnol., 35: 1503-1516 (2008).
- Sannasi, P., Salmijah, S., Kader, J. and Othman, O., Physical growth and biomass characterization of bacterial cells exposed to Cd(II), Cr(VI), Cu(II), Ni(II), and Pb(II). J. Environ. Res. Dev., 4(1): 8-18 (2009).
- Sannasi, P., Kader, J. and Salmijah, S., Isolation and selection of mixed cultures (environmental isolates) from metal contaminated areas. In the Proceedings of the 12th National Biotechnology Seminar. Lumut, Malaysia, 12-15 November 2000, 116-120 (2000).
- Malakul, P., Srinivasan, K.R. and Wang, H.Y., Metal toxicity reduction in naphthalene biodegradation by use of metal-chelating adsorbents. Appl. Environ. Microbiol., 64: 4610-4613 (1998).
- Kratochvil, O. and Volesky, B., Advances in the biosorption of heavy metals. Trends Biotechnol., 16: 291-300 (1998).
- American Public Health Association, Standard methods for the examination of water and wastewater, 18th Ed. Washington DC, USA, 730-750 (1992).
- Kader, J., Sannasi, P., Othman, O., Ismail, B.S and Salmijah, S., Removal of Cr (VI) from aqueous solutions by growing and non-growing populations of environmental bacterial consortia. Global J. Environ. Res., 1(1): 12-17 (2007).
- Ganguli, A. and Tripathi, A.K., Survival and chromate reducing ability of P.aeruginosa in industrial effluents. Lett. Appl. Microbiol., 28: 76-80 (1999).
- Environmental Quality Act and Regulations Handbook, Details on Environmental Quality Act 1974 and regulations amendments from 1980 to May 1996, Act 127. In: Laws of Malaysia. Kuala Lumpur: MDC Publishers, 34-54, 127-151 (1996).
- Hernandez, A., Mellado, R.P. and Martinez, J.L., Metal accumulation and vanadium induced multidrug resistance by environmental isolates of E.coli and E. cloacae. Appl. Environ. Microbiol., 64: 4317-4320 (1998).
- Basu, M., Bhattacharya, S. and Paul, A.K., Isolation and characterization of chromium-resistant bacteria from tannery effluents. Bull. Environ. Contam. Toxicol., 58: 535-542 (1997).
- Ehrlich, H.L., Microbes and metals. Appl. Microbiol. Biotechnol., 48: 687-692 (1997).
- Zahoor, A. and Rehman, A., Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci., 21(6): 814-820 (2009).
- Camargo, F.A.O., Bento, F.M., Okeke, B.C. and Frakenberger, W.Y., Chromate reduction by chromium-resistant bacteria isolated from soils contaminated with dichromate. J. Environ. Qual., 32: 1228-1233 (2003).
- Asheh, S.A. and Duvnjak, Z., Adsorption of metal ions by moss. Adv. Environ.Res., 1: 510-517 (1997).
- Sar, P., Kazy, S.K. and Singh, S.P., Intracellular nickel accumulation by Pseudomonas aeruginosa and its chemical nature. Lett. Appl. Microbiol., 32: 257- 261 (2001).
- Dhamotharan, R., Murugesan, S. and Yoganandam, M., Bioremediation of tannery effluent using cyanobacterium. Biosci., Biotechnol. Res. Asia, 4(1): 201-206 (2008).
- Jacques, R.J.S., Okeke, B.C., Bento, F.M., Peralba, M.C.R. and Camargo, F.A.O., Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour. Technol., 99: 2637-2643 (2008).
- Vidali, M., Bioremediation: an overview. Pure Appl. Chem., 73(7): 1163-1172 (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.





