How to Cite | Publication History | PlumX Article Matrix
Is A Safe Dose Of Aqueous Extract Of Azadirachta Indica Toxic On The Histology Of The Rabbit Liver?
R. E. Ucheya1* and J. C. Igweh2
1Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin-City Nigeria. 2Department of Physiology, University of Nigeria, Enugu Campus. Enugu Nigeria.
ABSTRACT: The effect of aqueous extract of Azadirachta indica (A. indica) on the liver was conducted using seven rabbits, with mean body weight ranging between 1.43kg and 1.47kg and were allowed an acclimatization period of two weeks. The rabbits were separated at random into two groups (A, and B). Identification of each animal was done by the number of strokes marked on their tail. The test group (A) was given 350mg/kg/bodyweight of aqueous extract of A.indica, while the second group (B) was the primary control group and as such was given normal saline. Each rabbit was weighed at an interval of five days before and after administration of the extract, in inorder to determine the effect on the body weight. On the fifteenth day after extract administration, the animals were euthanized and histological processing of the liver was done after which the Photomicrographs of the liver were produced and the histological changes were studied. The photomicrographs of the liver revealed normal histoarchitecture of the cells when compared to the control group and the difference in percentage weight change between the treated (0.68%) and control (1.4%)group was statistically insignificant (0.72%, P> 0.001). Therefore, we suggest that aqueous extract of A.indica has no histological effect on the liver and body weight when taken at a recommended safe dose.
KEYWORDS: Histological changes; liver; body weight; aqueous extract; Azadirachta indica
Download this article as:| Copy the following to cite this article: Ucheya R. E, Igweh J. C. Is A Safe Dose Of Aqueous Extract Of Azadirachta Indica Toxic On The Histology Of The Rabbit Liver?. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Ucheya R. E, Igweh J. C. Is A Safe Dose Of Aqueous Extract Of Azadirachta Indica Toxic On The Histology Of The Rabbit Liver?. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9186 |
Introduction
Azadirachtaindica,commonly known as “neem”and Dogoyaro tree in Nigeria,isoften used locally in treatment of malaria, diabetes, hypertension, toothaches and fungal infections. The leaves, used as medicine, are generally available year-round as the tree is evergreen except during severe hot climate or if exposed to frost.Previous studies have documented its traditional administration or dosage.Leaf extract gel or toothpaste, 1gram (1/5 of a teaspon)in the morning and at bed time brushed all over the mouth is said to be helpful in treatment of stomach ulcers (Bandyopadhyay et al., 2004).Creams containing 5% or more of neem oil or neem extracts are typically applied at least twice per day for skin or vaginal infections.Neem oil (in a concentration of 1 to 4 %) mixed in coconut, mustard, or other oil base is used for repelling insects (Okpanyi and Ezeukwv, 1981).The seed oil is more problematic and should be kept out of reach of children because of a report of Reye’s symptoms in a few infants who consumed 5ml of the oil and ultimately died (Sinniah and Baskaran,1981). Adults may sometimes have diarrhoea, nausea or stomach upset when taking neem oil (Caius and Mhaskae 1932).No deaths have been reported in adults, but because of the potential for severe adverse effects the amount of neem extract used should not exceed the amount that has been safely used in researched studies.Neem should be avoided in pregnancy until its safety is demonstrated (Melanie, 1998)
The above studies encompass the adverse effect of neem oil in children and its anti-ulcer effect, and advices to further check its safety on humans. However, this present study is aimed at investigating if the reported safe dose in mice is also safe in Rabbits,so that it can be employed as a reference pointto man.
Research Methodology
Soxhlet extractor (50ml capacity), Matttler weighing balance, dissecting instruments-set, tissue processing materials (Xylene, Formaline, Alcohol, Rotary microtome, Knife, Slides, Cover slips, Paraffin, and embedding mould, etc. Microscope with digital camera, film mountant. Test chemical-aqueous extract of neem.
Animals
A total of seven (7) rabbits were used for this study, they were obtained from Department of Physiology, university of Nigeria, Enugu Campus. The animals were divided into two groups A and B. Group A (treated animals) had five rabbits while group B (control animals)had 2 Rabbits. Each day, before and after administration of the extract, the weight of individual animals in groups (A & B) was recorded and the average weight determined.
Extract Preparation
The extract was prepared as follows: fresh leaves of A. indica (580 g) were thoroughly mashed in distilled water (2 L). The decoction was filtered using a clean sieve cloth. Its concentration was determined by evaporating the extract to dryness. Freshly prepared extracts were used. Concentration of the aqueous extract was found to be 15.25 mg mL–1 dry extract and pH was 5.2, indicating a weakly acidic extract. The extractive yield (expressed as dry mass of the extract relative to the mass weight of the leaves) was determined to be 4.2%. The extract was concentrated so as to prepare 100 mg mL–1 dry extract solution.
Extract Administration
The rabbits were administered aqueous extract of A. indica orally through a cannula inserted into the oral cavity. 350mg/kg/body weight was given to each animal in Group A (treated animals) while group B (control animals) were given only saline water and feed. The concentration given was based on the report that aqueous neem leaf extract is said to be safe at a concentration ≤400mg/kg/body weight (Ucheya and Anibeze, 2009).
Tissue collection
Two weeks after administration of the various extracts, the animal’sweights were taken. The living Rabbits in groups (A and B) were euthanized. The Liver of the dead animals was immediately obtained by dissection for histological preparation as to prevent post mortem changes. Small pieces of about 2mm thick were then taken for fixation and were fixed for about 24hrs in 10% changes of formalin. These organs were then processed by standard histological procedures.
Significance of the difference in weight between the control and test values was evaluated using Student’s t-test. This was done using the computer programme ‘Statistical Package for Social Sciences (SPSS)’, version 15.0. P < 0.05 was taken as the significance level and the photomicrographs of the kidney were observed for any morphological changes.
Assessment of Micrographs
Morphometric method: Measuring the parameter or physical features on the micrographs
to determine the existence of an abnormality by using the normal features of the control animals as a standard.
Comparism– General physical comparism by using control animals as a standard.
Chromographic method: Observation for any changes in coloration by using the normal
histological colour as a standard.
Derangement of cells:
(a). Vacualation – accumulation of water, lipid or glycogen inside the
Cells.
(b). Necrosis – counting of the number of cells e.g. Nuclei
(c). Inflammation – (i) Acute, usually 0 – 3 days, indicated by
appearance of neutrophils(ii) Chronic, usually within a period of two weeks, indicated by
presence of lymphocytes and monocytes.
Results
Table 1: Showing Effects of Administration of 350mg/kg/body weight of Aqueous extract of A.indica on the weight of the Rabbits.
| Animal
Group |
Extract
Administered |
Mean Weight
Before Extract Administration (Kg) (a) |
Mean Weight at
5th day of Drug Administration (kg) |
Mean Weight at
10th day of Drug Administration (Kg) |
Mean Weight at
15th day of Drug Administration (kg) (b) |
Mean
Weight Difference (kg) (b-a) |
Percentage
Weight Gained (kg) |
| A | Aqueous
Extract of A.indica |
1.46±1.2 | 1.46 ± 3.1 | 1.45 ± 2.1 | 1.47 ± 2.3 | 0. 01 ± 2.3 | 0.68% |
| B | Control | 1.43 ± 1.4 | 1.44 ± 2.2 | 1.44 ± 3.1 | 1. 45 ± 2.4 | 0.02 ± 3.4 | 1.40% |
The table above shows that the Percentage difference between the treated group of animals and control group (0.72%) is statistically insignificant using students t-test (P>0.001).
Histological Effects of A. indicaon the photomicrographs of the Liver Tissue
The photomicrographs of the liver tissue of the control animalsrevealed a normal histoarchitecture (Figure 1). The photomicographs of the treated animals treated with 350mg/kg body weight of aqueous extract of A. indica also reveals a normal histoarchitecture of the liver cells (Figure 2),this was evidenced by normal size and apperance of the sinusoidal cells which were very visible on the photomicrographs (Figures 1 & 2).
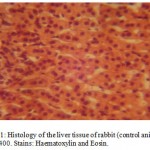 |
Figure 1:Histology of the liver tissue of rabbit (control animals) Mag.×400. Stains: Haematoxylin and Eosin.
|
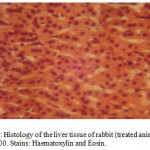 |
Figure2:Histology of the liver tissue of rabbit (treated animals) Mag.×400. Stains: Haematoxylin and Eosin.
|
Discussion
The current investigation reveals that A. indicawhen taken at a recommended safe dose has no effect on the weight of rabbit (table 1), this is consistent with anti-oxidant theory that Herbal products when taken in its natural form and dosage have no harmful effect (Okazaki, et al., 2007). Secondly, it agrees with the findings of Melanie, (1998) that the major concern with the use of herbals medication is administration of an overdose, batch-batch variability and are not regulated for purity. This finding is also in conformity with the report by Ucheya and Anibeze, (2009) that Aqueous extract of A. indicais safe on the mice liver when administered at an appropriate dose (≤ 400mg/kg/body weight), but unsafe when administered at an overdose
> 400mg/kg/bodyweight).This also is consistent with our present findings as evident in the normal histoarchitecture of the rabbit liver (Figures 1 & 2).
Conclussion
We suggest that a safe dose (≤ 350 mg/kg body of Rabbit) of A.indica extract should be administered when used as a curative agent for any disease.
References
- Bandyopadhyay U., Biswas K., Sengupta. (2004). Clinical studies on the effect of Neem (Azadirachtaindica) Bark extract on gastric secretion and gastroduodenal Ulcer. Life Sci. 75: 2867-76.
- Caius J.J., Mhaskae, V.P. (1932). USDA Interest in proceedings of a workshop on neem’spotential in pest management programs. USD-ARS, Beltsville, MD.ARS-86.1-3.
- Sinniah D., baskaran G., (1981).The effect of neem oil poisoning as a cause of Reye’s
- syndrome. Lancet. 28: 487-489.
- Malanie J.C. (1998). Herbal Remedies: Adverse effects and drug interactions. American Academy of family physicians.(Clinical Pharmacology). 31:33-37.
- Okazaki K., Sakamoto K., Kikuchi R., Togashi E., Kuginuki Y., Matsumoto S., Hirac M.(2007). TheorAppl Genet., 144 (4): 595-608
- Okpanyi S.N.,G.C.Ezeukwv., (1981).Antiinflammatory and anti-pyretic effects of A.indica.Planta Med. 41-39.
- Ucheya R.E., Anibeze CIP., (2009). Histological Changes in mice organs administered with various concentrations of neem extracts (Azadirachtaindica).Biosciences, Biotechnology Research Asia, Vol.06 (02) in press.

This work is licensed under a Creative Commons Attribution 4.0 International License.





