How to Cite | Publication History | PlumX Article Matrix
Isolation of Soil Bacteria for Bioremediation of Hydrocarbon Contamination
K. Moorthy et al
1Department of Microbiology, Vivekanandha College of Arts and Sciences for Women, Elayampalyam, Tiruchengode- 637 205 India.
2Department of Biotechnology, Vivekanandha College of Arts and Sciences for Women, Elayampalyam, Tiruchengode - 637 205 India.
3Department of Bioinformatics, Vivekanandha College of Arts and Sciences for Women, Elayampalyam, Tiruchengode - 637 205 India.
4Department of Microbiology, Bharathidasan University, Tiruchirappalli - 620 024 India.
Corresponding Author E-mail:moormicro@gmail.com.
ABSTRACT: The study was conducted to identify the capability of the bacterial strains to degrade the crude oil in in vitro conditions. The soil samples were collected from oil contaminated sites at Aravakurichy, Karur district, Tamilnadu. Soil samples were used to analyze the moisture content, temperature, pH, total nitrogen, phosphorous, BOD and COD. From the soil sample , three dominant bacterial strains were isolated viz, Bacillus subtilis, Pseudomonas aeruginosa, and KIebsiella pneumoniae. The isolates were screened for its ability to degrade crude oil and all the isolates showed maximum degradation. The degrading ability was higher in liquid medium when compared to solid media. Among the three isolated bacterial strains, P. aeruginosa showed higher degrading ability than the other two isolates. A detailed analysis of the hydrocarbon extract was performed by Gas Chromatography.
KEYWORDS: Xenobiotics; bioremediation; hydrocarbon soil bacteria
Download this article as:| Copy the following to cite this article: Moorthy K. Isolation of Soil Bacteria for Bioremediation of Hydrocarbon Contamination. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Moorthy K. Isolation of Soil Bacteria for Bioremediation of Hydrocarbon Contamination. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9441 |
Introduction
The increasing release of organic pollutants by industries cause many health related problems. Polycyclic aromatic hydrocarbons are the ubiquitous oil pollutants. Crude oil can be accidentally or deliberately released into the environment leading to serious pollution problems (Thouand et al., 1999). These pollution problems often results in huge disorder of both the biotic and abiotic components of the ecosystem (Mueller et al.,1992). Even some of the hydrocarbon components are highly toxic, mutagenic or carcinogenic in nature (Hallier et al., 1999). However increased awareness of harmful effects of environmental pollution has led to a dramatic increase in research on various strategies that may be employed to clean up the environment. The process leading to the eventual removal of hydrocarbon pollutants from the environment has been extensively documented and involves physical, chemical and biological alternatives. Biodegradation of the hydrocarbons by natural populations of microorganisms has been reported to be the main process acting in the depuration of hydrocarbon-polluted environments (Challain et al., 2006), the mechanism of which has been extensively studied and reviewed (van Hamme et al., 2003).
Hydrocarbon is an organic compound consisting entirely of Hydrogen and carbon. Hydrocarbons are mainly the pollutants from oil refineries and oil spills. Hydrocarbons contain benzene, cyclopentadiene, dicylopentadiene, styrene, toluene and xylene as major components and many other hydrocarbons as minor components. The majority of the hydrocarbons are found in naturally occurring crude oil, diesel and petroleum [Santhini, et al., 2009].
The bioremediation of the soil contaminated with petroleum and its products depends on the number of hydrocarbon-degrading microorganisms in the soil. A single microorganism can degrade only certain types of petroleum compounds, but a mixed microbial community enables the higher levels of degradation. Many microorganisms have the ability to utilize hydrocarbon as the sole source of carbon and energy. Such microorganisms are widely distributed in nature. The ability of microbes to degrade organic contaminants into harmless constituents has been explored as a means to treat the polluted environments biologically [Sonakya et al 2007].
The present study deals with isolation and identification of the bacterial strains from hydrocarbon contaminated soil to assess their potential for bioremediation.
Materials and Methods
Sample collection
The sample was collected from the crude oil polluted soil in a petrol bunk at Aravakurichy, Karur district. They were collected aseptically using sterile soil sampler and stored in aluminium foil then transported to the laboratory within 24 hours of collection.
Enumeration & isolation of hydrocarbon utilizing bacteria:
The crude oil contaminated soil sample was serially diluted. From the diluted sample (10-3 ) 0.1 ml of the sample was inoculated into nutrient agar to enumerate the total number of bacteria and then inoculated the pre-dried mineral salt agar. A filter paper saturated with sterile crude oil was aseptically placed above the medium , pressed gently and were incubated for four days at 37oC . The plates yielding 30-250 colonies were enumerated for the hydrocarbon utilizing bacteria and the isolates were identified by Standard procedure.
Sample Analysis
Analysis of physicochemical parameters of the soil sample
The crude oil contaminated sample was analyzed for various Physico-chemical analysis viz., Colour of the sample, Odour (direct smelling of the sample), temperature measurements (Standard thermometer was used) and pH of the sample ( determined by pH meter).
ADMI color was determined with a Spectrophotometer in accordance with the ADMI Tristimulus method (2120 D) detailed in Standard Methods. The spectrophotometer was calibrated before each use with standard platinum cobalt color solutions of 100, 200, 300, 400, and 500 ADMI color units. Phosphorous content was determined by Olsen method 1987. Nitrogen content was determined calorimetrically in accordance with Kjeldahl method, BOD and COD were done by Wrinkler’s method.
Degradation Analysis
The isolated bacterial strains were inoculated into the plates containing crude petroleum. Degradation of the hydrocarbon was visually observed for the clearance of zone on the plates.
Bacterial isolates were inoculated in 50 ml of broth for overnight at 37o C – 80 rpm until the OD value reaches 1.00 at 600nm. The cultures were centrifuged at 10,000 rpm for 10 min. and washed twice with sterile saline (0.85%) and resuspended in 10 ml of saline solution. 0.1ml of the inoculum was added to the broth containing crude oil and incubated at 37°C, 85 – 110 rpm for 24 hr. The supernatant was collected after centrifugation for absorbance measurement at respective wavelengths. The percentage of degradation was calculated as follows:
Analyses of extract: (Gas Chromatography)
A detailed analysis of the hydrocarbon extract was performed by Gas Chromatography (GC). The clarus GC 500 was equipped with a split injector (split ratio 50/1) and a Flame Ionization Detector FID both set at 300°C, gas carrier was nitrogen 1.50 ml min−1, the column was fused silica capillary column (30.0 mx0.32 mm, film thickness 0.25µm), temperature programming was 60- 320°C, 5°C min−1, injection volume 1µl.
Results
The total bacterial counts in crude oil polluted soil sample ranged from 39 cfu/0.1 ml while in the control (crude oil free plates) the counts ranged 45 cfu/ 0.1 ml. Generally the colony counts were higher in crude oil free soil than in crude oil polluted soil which are shown in the Table 1.
Table 1: Enumeration of total and Hydrocarbon utilizing bacteria in the soil samples:
| S.No. | Sample | Bacterial Counts (cfu/0.1 ml) |
| 1. | Oil contaminated soil | 39 |
| 2. | Hydrocarbon utilizing bacteria | 36 |
| 3. | Oil free soil | 45 |
The hydrocarbon utilizing bacterial isolates were identified based on the biochemical characterization. The biochemical results revealed three isolates such as Bacillus subtilis, Pseudomonas aeruginosa and Klebsiella pneumonia. The moisture content of the soil sample was noted in equal time intervals and the results were recorded in Table 2.
Table 2: Moisture content
| S.No. | Time (hours) | Moisture content(gram) |
| 1. | 0 | 15 |
| 2. | 2 | 14.2 |
| 3. | 4 | 12.6 |
| 4. | 6 | 11.5 |
| 5. | 8 | 11.0 |
| 6. | 10 | 10.3 |
The physicochemical characteristics of soil sample collected from petrol bunk used for the study analyzed and tabulated in Table 3. The available phosphorus level of crude oil polluted soil were found as 24.09 mg/kg was higher than that of crude oil free soil 18.14 mg/kg. The Nitrogen content of the crude oil polluted soil was found as 12.5 mg/kg were higher than that of crude oil free soil 10.45 mg/kg. The BOD of the crude oil polluted soil was found as 0.9 mg/l were higher than that of crude oil free soil 0.8 mg/l. The COD of the crude oil polluted soil was found as 1.6mg/l were slightly equal to that of crude oil free soil 1.2 mg/l and were tabulated as physicochemical parameters of crude oil contaminated soil in Table 3
Table 3: The Physicochemical parameters of the crude oil contaminated soil
| S.No. | Physicochemical Parameters | Readings |
| 1 | pH | 8.5 |
| 2 | Temperature | 50 0 C |
| 3 | Moisture | 15 – 10.3 |
| 4 | Phosphate | 24.09 mg/kg |
| 5 | Nitrogen | 12.5 mg/kg |
| 6 | BOD | 0.9 mg/l |
| 7 | COD | 1.6 mg/l |
Analysis of Degradation
The isolated bacterial samples Bacillus subtilis, Pseudomonas aeruginosa and Klebsiella pneumoniae were checked for the extent of crude oil degradation both in solid medium as well as in liquid medium. All the isolates given maximum degradation. Visual degradation indicated that degradation was higher in case of basal broth when compared than minimal medium.
The overnight broth cultures (B. subtilis, P.aeruginosa and K.pneumoniae ) with crude oil were centrifuged and resuspended in 10 ml of saline solution and 0.1ml of the inoculum was added to the broth containing crude oil and incubated at 37°C, 85 – 110 rpm for 24 hr. The supernatant was collected after centrifugation for absorbance measurement at 650 nm wavelengths. Among the three isolated bacterial strains, P.aeruginosa showed higher degradation than other two isolates. The percentage degradation was calculated and given in Table 4 and Figure 1.
In of the process conditions for more efficient application of biological degradation of oil pollutants under different climatic conditions and other diverse environmental conditions.
Table 4: Analysis of Degradation
| S.No | Bacterial cultures | Percentage of Degradation (%) |
| 1 | Bacillus subtilis | 65 |
| 2 | Pseudomonas aeruginosa | 73 |
| 3 | Klebsiella pneumoniae | 52 |
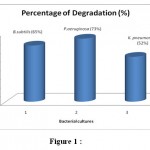 |
Figure 1
|
Discussion
Bioremediation is a promising technology for the treatment of a wide range of contaminants in soil and groundwater. The method is cost-effective, particularly for procedures dealing with petroleum hydrocarbon contamination, and can be easily integrated with other remedial technologies.
The currently accepted disposal methods of incineration or burial in secure landfills (USEPA, 2001; ITOPF, 2006) can become prohibitively expensive when the amounts of contaminants are large. This often results in cleanup delays while the contaminated soil continues to pollute groundwater resources if on land, and death of aquatic life if on waterways (Pye and Patrick, 1983), thus necessitating speedy removal of the contaminants. Biodegradation of petroleum is an important alteration process with major economic consequences for oil and gas production. Evidence is emerging to support the hypothesis that in reservoir petroleum, biodegradation is caused by anaerobic hydrocarbon degrading bacteria (Aitken et al., 2004).
With an understanding of the hydrocarbon degradation process of microbes in the environment, it is possible to develop strategies for utilizing microbial hydrocarbon degrading activities to remove the hydrocarbons from contaminated ecosystems.
The present study mainly focuses on the isolation & identification of the bacterial strains from hydrocarbon contaminated soils to assess their potential for bioremediation. The use of a plate screening technique allowed the direct isolation and quantification of polycylic aromatic hydrocarbon (PAH)-degrading bacteria from different soil sites (Kastner, et al.,1993). The count of total bacterial counts in crude oil polluted soil sample ranged from 39 cfu/0.1 ml while in the control (crude oil free plates) the counts ranged 45 cfu /0.1 ml. Generally bacterial counts in the both polluted and free soil where higher than the fungal counts in both soils (Ijah et al., 2003). The higher counts of bacteria compared to fungi may be as a result of the nutrient status of the soil (Jobson et al., 1974) and the presence of some toxic components which do not favour fungal growth (Colwell and Walker, 1977).
Temperature plays very important roles in biodegradation of petroleum hydrocarbons, primarily by its direct effect on the chemistry of the pollutants, and secondly on its effect on the physiology and diversity of the microbial population. Ambient temperature of an environment affects both the properties of spilled oil and the activity or population of microorganisms (Venosa and Zhu, 2003). At low temperatures, the viscosity of the oil increases, while the volatility of toxic low-molecular weight hydrocarbons is reduced, delaying the onset of biodegradation (Atlas, 1981).In the crude contaminated soil, the temperature ranges at 500 C that favours the growth of the microorganisms and the degradation process
The pH of the crude oil contaminated soil is 8.5. The slight alkaline pH of water seems to be quite favourable for petroleum hydrocarbon degradation, but in acidic soils limiting to pH 7.8 – 8.0 had a definite stimulatory effect (Anthony I Okoh, 2006). Both Nitrogen and phosphorous levels were higher in crude oil polluted soil than crude oil free soil. This agrees with the finding of (Odu, 1972) who reported increase in nitrogen and phosphorous content of a crude oil polluted soil. Nutrients are very important ingredients for successful biodegradation of hydrocarbon pollutants, especially nitrogen, phosphorus and in some cases iron (Cooney, 1984).
Typical bacterial groups known for their capacity to degrade hydrocarbons include Pseudomonas, Marinobacter, Alcanivorax, Microbulbifer, Sphingomonas, Micrococcus, Cellulomonas, Dietzia, and Gordonia groups (Brito et al., 2006). In this study the hydrocarbon utilizing bacterial isolates were identified based on the biochemical characterization. The biochemical results revealed three isolates such as Bacillus subtilis, Pseudomonas aeruginosa and klebsiella pneumoniae. The rate of crude oil biodegradation in the soil seems to be rapid. This may be due to fact that the microorganisms in the soil some have efficient ability in utilizing the residual crude oil as a source of carbon and energy (Ijah and Antai, 2003).
Much need still exist for the optimizatio
Reference
- Thouand G, Bauda P, Oudot J, Kirsch G, Sutton C, and Vidalie J.F., Laboratory evaluation of crude oil biodegradation with commercial or natural microbial inocula. Can. J. Microbiology, 45 (2) : 106-115 (1999).
- Mueller J.G., Resnick S.M., Shelton M.E., and Pritchard P.H., Effect of inoculation on the biodegradation of weathered Prudhoe Bay crude oil. J. Indstustrial Microbiology, (10) : 95-102 (1992).
- Hallier-Soulier S , Ducrocq V, Mazure N., and Truffaut N., Detection and quantification of degradative genes in soils contaminated by toluene. FEMS Microb. Ecol., (20) : 121-133 (1999).
- Chaillan F., Chaineau C.H., Point V., Saliot A., and Oudot J., Factors Inhibiting bioremediation of soil contaminated with weathered oils and Drill cuttings. Environmental Pollution, 144 (1) , 255-265 (2006).
- Van Hamme J.D., Singh A., and Ward O.P., Recent advances in petroleum microbiology. Microb. Mol. Biol., Rev., 67 : 503-549 (2003).
- Santhini. K., Myla.J., Sajani.S., and Usharani.G., Screening of Micrococcus sp. from oil contaminated soil with reference to Bioremediation. Botany Research International, 2 (4) : 248-252 (2009).
- Sonakya.V., Raizada.N., Hausner.M., and Wilderer.P.A., Microbial populations associated with fixed and floading-bed reactors during a two stage anaerobic process. Int Microbiol., 10 : 245-251 (2007).
- Pye, V.I., Patrick, R., and Quarles. J., Groundwater contamination in the United States. Science. 221 : 713-718 (1983).
- Aitken. C.M, Jones,D.M., and Larter,S.L., Anaerobic hydrocarbon degradation in deep subsurface reservoirs. Nature, 431 : 291-294 (2004).
- Kastner, M. Breuer-Jammali and Mahro, B., Enumeration and characterization of the soi l microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) . Applied Microbial and Cell Physiology, 41 (2) : 267-273 (1993).
- Ijah. U.J.J, and Abioye, O.P., Assessment of Physicochemical and Microbiological properties of Soil 30 months after Kerosene Spill, J. Res. Sci. Manage, 1: 24-30 (2003).
- Jobson. A.M., McLaughlin,F.D., and Westlake,D.W., Effects of Amendment on Microbial Utilization of Oil Applied to Soil. J. Applied Microbial, 27 : 166-170 (1974).
- Colwell. R.R, and Walker, J.D., Role of Autochthonous Bacteria in the Removal of spilled Oil from Sediments. Environmental Pollution, 12 : 51-56 (1997).
- Venosa A.D., and Zhu X., Biodegradation of Crude Oil Contaminating Marine shorelines and Freshwater Wetlands. Spill Sci. Tech. Bull. 8 (2) : 163-178 (2003).
- Atlas RM., Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microb. Rev. 45 : 180-209 (1981).
- Anthony I Okoh., Biodegradation alternative in the cleanup of petroleum hydrocarbon pollutants. Biotechnology and Molecular Biology Review 1 (2), 38-50 (2006).
- Odu. T.K., Microorganisms and Petroleum Pollutants. Bioscience, 28 : 387-369 (1972).
- Cooney J.J., The fate of petroleum pollutants in freshwater ecosystems. K 03099 Pollution; J 02905 Water; P 2000 Freshwater Pollut. (1984).
- Brito, E. M. S., Guyoneaud, R., Goni-Urriza, M. S., Ranchou-Peyruse, A., Verbaere, A., Crapez, M. A. C., Wasserman, J. C. A. and Duran, R., Characterization of hydrocarbonoclastic bacterial communities from mangrove sediments in Guanabara Bay, Brazil. Research in Microbiology, 157(8) : 752-762 (2006).
- Ijah. J, and S.P Antai, Degradation and Mineralization of Crude oil by Bacteria. Lett. Applied Microbial, 12 : 72-76 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.






