How to Cite | Publication History | PlumX Article Matrix
Optimization of Media, Growth Factors and Substrate for Methylobacterium
S. Meenakumari1 and S. Ahmed John2
1Department of Biotechnology, N.M.S.SVN College (Autonomous), Madurai - 625 019 India.
2Post Graduate and Research Department of Botany Jamal Mohammed College, Tiruchhirappalli - 620 020 India.
ABSTRACT: Methylobacterium, a group of organism which oxidizes methane and C1 carbon compounds. Some of these organism forms pink color colonies when they are cultivated in selective media, called Ammonium Mineral Salt Medium. The present investigation is mainly focused to optimize their growth factors, like PH, Temperature, Media modulation and Substrate optimization and their inhibitors of growth. Because Methylobacterium have fertilizer value and they also act as potential Bioinoculant for improving crop production.
KEYWORDS: Methylotrophs; Methane; C1 Carbon compounds; Bioinoculant; Inhibitors
Download this article as:| Copy the following to cite this article: Meenakumari S, John S. A. Optimization of Media, Growth Factors and Substrate for Methylobacterium. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Meenakumari S, John S. A. Optimization of Media, Growth Factors and Substrate for Methylobacterium. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9787 |
Introduction
There are number of microorganisms that utilize single carbon substrates as sole energy sources. These organism are referred to as Methylotrophs are herein as C1 “metabolisers” (McDonald etal.,1995).These organism are characterized by the ability to use carbon source of energy and biomass (Basile, et al 1985). Methylotrophs are ubiquitous in nature, it can be readily isolated from leaf disks or whole leaves of plant or soil sample. Corpe,W A (1985) suggested that the growth of organism was rich in methanol salt agar and predominant methylotroph encountered and isolates were pigmented, Facutatively methylotrophic (PPFM) bacteria. Methylobacterium can utilize a broad range of substrate(Antony.,1986) include Methanol, Ethanol, Fumarate, Acetate, Acetone, Pyruvate, Glycerol. Organism can grow up to 1ml/100ml where as succinate, fumarate and pyruvate can be given as 10mM. growth can be monitored by measuring optical density at 620 nm.(Wood et al.,1971)
Materials and Methods
Soil sample from various places were collected (Paddy field, Ditch soil, Distillery sludge). It was serially diluted in selective media. Serially diluted samples were then subjected to morphological characteristics
identification. colonies were selected based on the pigmentation. Gram staining, citrate and oxidase test also peformed. The colonies were then cultured in medium containing glycerol and penicillin. The cultivated organism was kept as pure culture. Sub culture was prepared and stored. The sub cultured organism was subjected to Optimization of Growth factors includes PH and Temperature. Results were identified and tabulated. Growth curve of the organism was drawn with the optimized factors. Medium were prepared with various substrates which includes, Glycerol, Ethanol, Fumarate, Methanol, Pyruvate, Acetate, Acetone. Growth was monitored at regular time intervals and the results were tabulated.
 |
Photo 1: Citrate positive.
|
 |
Photo 2: Oxidase positive.
|
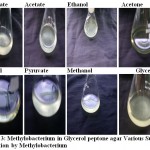 |
Photo 3: Methylobacterium in Glycerol peptone agar Various Substrate utilization by Methylobacterium.
|
Results and Discussion
Methylobacterium are ubiquitous in nature they can be found anywhere. Primary screening of the organism using various soil sample from various places were done. It was observed that some organism produced pink color colonies. Colony morphology showed, circular with unbroken peripheral edge with entire sharply defined margin and had dome shaped elevation. The staining shown that is was a gram negative short rod and biochemical test showed positive to citrate (Photo-1) and oxidase (Photo-2). Though it is a methane utilizer, it has the ability to utilize C1 compuonds. Keeping this view, various factors were optimized. Using different media, modified glycerol peptone agar supplemented with antibiotic penicillin and glucose. In the presence of these two compounds the organism grown well because antibiotic inhibits the growth of other gram positive organism where as glucose is a carbohydrate source (Photo-3). PH were optimized by adjusting the AMS broth to various PH. The result showed at PH 7-and 7.4, the growth was optimum (Figure-1).Temperature also optimised by incubating culture at various temperature for specific duration. It was revealed that at 36C, the growth was optimum (Figure-2). Substrate utilization was checked by supplementing substrate in AMS medium. Growth was monitored for 22 hours. It was found that organism effectively utilizes fumarate (Figure -3). But the growth was maximum in the presence of methanol. The present investigation found that the organism can easily oxidizes methanol, for the production of cellular components.
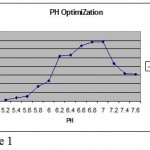 |
Figure 1
|
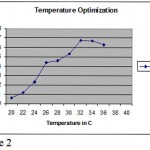 |
Figure 2
|
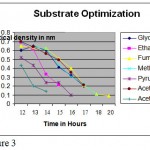 |
Figure 2
|
References
- Antony,C.1986. Bacterial oxidation of methane and methanol. Adv.Microbiol.Physiol 27:113-210
- Basile, DV., Basile,M.R., Li,Q.Y and Corpe,W.A 1985. The Bryologist .88:77-81
- Corpe,W.A 1985 Methylotrophs are ubiquitous in nature. Journal of Microbiological Methods Vol-3:3-4
- Mc Donald,I.R., E.M.Kenna, and J.C.Murrell.1995. Detection of Methanotrophic bacteria in Environmental samples with the PCR. Appl.Environ.Microbiol-61:116-121
- Wood,P.J., and Siddique 1971. Determination of Methanol and its application to measurement of pectin ester content and pectin methyl esterase activity.Anal.Biochem.39:418-428

This work is licensed under a Creative Commons Attribution 4.0 International License.





