How to Cite | Publication History | PlumX Article Matrix
M. Prabhakaran1*, S. M. Sundarapandian2 and S. Elumalai1
1PG and Research Department of Plant Biology and Biotechnology, Presidency College, Chennai - 600 005 India.
2Department of Ecology and Environmental Sciences, School of Life Sciences, Pondicherry University, Puducherry - 605 014 India.
Corresponding E-mail:prabhasnc@gmail.com
ABSTRACT: The present investigation is intended to evaluate the phytochemical characters such as determination of pharmacognostic and screening of bioactive principles and antibacterial activity of an important medicinal plant, Odina wodier Roxb., Various parts of the plant Odina wodier, Roxb., family, Anacardiaceae, is traditionally used in Indian medicine. Phytochemical screening was carried out to assess the qualitative chemical composition of crude extracts using commonly employed precipitation and coloration reaction to identify the major natural chemical groups such as alkaloids, saponins, steroids, phenolic compounds, flavonoids, tannins, reducing sugars and amino acids. The microbes selected for the present study were Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus faecalis. They were obtained from research laboratory, Department of Microbiology, Thiagarajar College, Madurai, Tamil Nadu, India.In comparison with three parts of plant (Odina wodier) studied, (leaf, bark and root), root is better in terms of antibacterial action compared to bark and leaf. The present study concludes that the extracts of the root of Odina wodier, Roxb., has great potential as antimicrobial compound against pathogenic microorganisms studied and that it can be used in the treatment of infectious diseases caused by bacteria. This part of the plant can be used to discover bioactive natural products that may serve as leads for the development of new pharmaceutical drugs that address hitherto unmet therapeutic needs.
KEYWORDS: Phytochemical screening; antibacterial activity; Odina wodier
Download this article as:| Copy the following to cite this article: Prabhakaran M, Sundarapandian S. M, Elumalai S. Phytochemical Studies and Antibacterial Activity of the Leaf, Bark and Root Powders of Odina Wodier Roxb. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Prabhakaran M, Sundarapandian S. M, Elumalai S. Phytochemical Studies and Antibacterial Activity of the Leaf, Bark and Root Powders of Odina Wodier Roxb. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9392 |
Introduction
Plants have a great potential for producing new drugs of great benefit to mankind. Nowadays a renewed interest in traditional medicine is observed and there has been an increasing demand for more and more drugs from plant sources. This revival of interest in plant-derived drugs is mainly due to the current widespread belief that “green medicine” is safe and more dependable than the costly synthetic drugs many of which have adverse side effects. The need of the hour is to screen a number of medicinal plants for promising biological activity (Parekh and Chanda, 2006). Odina wodier, Roxb., has a medium sized to large tree 12-28 m in height with grayish bark rough, exfoliating in thin irregular flakes: leaves imparipinnately compound, crowded at the ends of branches, leaflets membranous, 7-9 oblong-ovate, green above, brown beneath glabrous, base acute or rounded often oblique, main nerves 6-8 pairs: flowers small, yellowish or purplish, the male racemes compound, the female simple; fruits reniform, compressed 1 – seeded red drups. Eng : Wodier, Jhingam. Tam : Oti, Udi, Vodiyar (Warrier et al., 1995). Bark of Odina wodier contains tannin and ash contains considerable quantity of potassium carbonate. Decoction of the bark (1in 10) is given as astringent in doses of ½ to 1 ounce, in cases of a tonic dyspepsia and general debility, particularly if combined with tincture of gentian, calumba, etc. It is also for tooth ache and as a lotion for skin eruptions. Fresh juice of the bark is a valuable application to sore eyes and obstinate ulcers. Bark powdered and mixed with neem oil is an application for chronic ulcers and skin diseases as impetigo. Powdered bark is used as a paste for leprous ulcers. Gum of the tree made into a liniment with brandy is a good application to sprains and bruises. Internally, gum is given in asthma and as a cordial to women during lactation. Leaves boiled in oil are also applied to sprains and bruises, to local swellings and pains of the body for rheumatism a paste of the leaves mixed with black pepper is a useful application. Juice of the green branches in 4 ounce doses, mixed with 2 ounces of tamarind is given as an emetic in cases of coma or insensibility produced by opium or other narcotic (Nadkarni, 1998).
Materials and Methods
Collection of materials
As per information given by the Siddha medical practitioners the plant material was collected at Perungudi village in Madurai district in the month of January 2008. The plant parts were used and the local names were recorded. The identification was carried out with the help of the Flora of the Presidency of Madras, Gample.
Preparation of plant extracts
The plant materials were washed with water to remove the adhering dust particles and were shade dried at room – temperature. Extracts were prepared from shade-dried samples. The dried plant materials were ground into a fine powder in an electric blender and subsequently sieved using a sieve for obtaining fine powder. Thereafter 5g each of fine powdered sample was weighed and soaked separately in 15 ml of different solvents (Absolute alcohol, Acetone, Benzene, Chloroform, Ether and Water) in the ratio of 1:3 weights for volume (W/V). These were allowed to stand for 24 hrs at ambient room temperature. The soaked plant powder was filtered through filter paper (Whatman No.1) and the filtrate was used as crude extract. Different crude extracts of these plants were stored in refrigerator and used as such for qualitative phytochemical analysis and for antibacterial assay.
Phytochemical screening
Phytochemical screening was carried out to assess the qualitative chemical composition of crude extracts using commonly employed precipitation and coloration reaction to identify the major natural chemical groups such as alkaloids, saponins, steroids, phenolic compounds, flavonoids, tannins, reducing sugars and amino acids. General reactions in these analyses revealed the presence or absence of these compounds in the crude extract were tested (Brinda et al., 1981). Table 1.
Table 1: Phytochemical screening test for the presence or absence of secondary metabolites (Brindha et al., 1981).
| S.No. | Test | Observation | Inference | |
| 1. | Test solution + acetic acid. Decant and added few drops of Dragendroff’s reagents | Yellow or Orange precipitate | Presence of alkaloids | |
| 2. | Test solution +2ml of anthrone reagent and heated then cooled | Dark green colour | Presence of anthrone glucosides | |
| 3. | To an alcoholic solution of test solution added a bit of magnesium and one or two drops of Conc. HCl and heated | Red colour or Orange red colour | Presence of flavonoids | |
| 4. | Alcoholic solution of test solution + one drop of ferric chloride
(or) To a drop of the solution on a filter paper added ferric chloride and ferric cyanide solution |
Intense colour
Blue colour develops
|
Presence of phenolic group
|
|
| 5. | Test solution + 2ml of Fehling’s reagent + 3ml of H2O and boiled | Red orange colour | Presence of reducing sugars | |
| 6. | Test solution + H2O | Foamy lather | Presence of Saponins | |
| 7. | Test solution + minimum CHCI3 + 3- 4 drops of acetic anhydride and one drop of Conc. H2SO4 | Purple colour changing from blue to green | Presence of Steroids | |
| 8. | Water-soluble portion of the extract treated with basic lead acetate solution | White precipitate | Presence of tannins | |
Antibacterial assay
Collection of microorganisms
The microbes selected for the present study were Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Proteusvulgaris, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus faecalis. They were obtained from research laboratory, Department of Microbiology, Thiagarajar College, Madurai, Tamil Nadu, India.
Preparation of inoculum
Each organism was recovered for testing by sub-culturing on fresh media. A loopful inoculum of each bacterium was suspended in 5 ml of nutrient broth and incubated overnight at 37oC. These overnight cultures were used as inoculum.
Preparation of media
The growth media employed in the present study included nutrient agar and nutrient broth. The medium was adjusted to pH 7.4 and sterilized by autoclaving at 15 lbs pressure (121oC) for 15 minutes.
Sub-culturing of microorganism
The pure cultures of microorganism were maintained on nutrient agar slants by frequent sub-culturing. These cultures were stored at 4°C.
Antibacterial activity
Antimicrobial activity was demonstrated by modified method described by Langfield et al. (2004). 0.1 ml of the diluted microbial cultures was spread on sterile nutrient agar plate. The soaked and dried discs of 6 mm diameter of Whatman filter paper No.1 were then placed on the seeded plates and gently pressed down to ensure contact. Four replicates were placed for each extracts in each solvent to confirm the inhibition zone. The plates were incubated at 37oC for 24 hours. After the incubation period the inhibition zone around the discs were measured and recorded as the difference in diameter between the disc (6 mm) and growth free zone.
Results and Discussion
Phytochemical screening of crude extracts
The screening of phytochemical components of the medicinal plant of Odina wodier, Roxb., is presented in Table 2.
Table 1: Phytochemical screening test for the presence or absence of secondary metabolites (Brindha et al., 1981).
| Extracts | Plant parts | Presence (+) or absence (-) of bio active compounds | |||||||
| Alkaloids | Anthrone glucosides | Flavonoids | Phenolic group | Reducing sugar | Saponins | Steroids | Tannins | ||
| Acetone | Leaf | – | – | + | – | – | – | – | – |
| Bark | – | – | + | + | + | – | – | + | |
| Root | – | – | + | + | – | – | – | + | |
| Benzene | Leaf | – | – | – | – | – | – | – | – |
| Bark | – | – | + | – | – | + | + | – | |
| Root | – | – | – | – | – | + | – | + | |
| Chloroform | Leaf | – | – | – | – | + | – | – | – |
| Bark | – | – | – | – | + | + | + | – | |
| Root | – | – | + | – | + | – | – | – | |
| Ethanol | Leaf | – | – | + | – | – | – | – | – |
| Bark | – | – | + | + | – | – | – | + | |
| Root | – | – | + | + | – | – | – | + | |
| Ether | Leaf | – | – | – | – | – | – | – | – |
| Bark | – | – | + | – | – | – | – | – | |
| Root | – | – | + | – | – | – | + | – | |
| Water | Leaf | – | – | + | + | – | – | – | + |
| Bark | – | – | + | + | – | + | – | + | |
| Root | – | – | + | + | – | + | – | + | |
Flavonoids
Flavonoids are present in the acetone, ethanol and water extracts of leaf, bark and root powders. Chloroform and ether extracts of root powder also contain flavonoids. Similarly benzene and ether extracts of bark powder only contain flavonoids. (Brindha et al., 1981).
Phenolic compounds
Phenolic compounds are present in the acetone and ethanol extracts of both bark and root powders. However, aqueous extracts of all the three parts powders contain phenolic compounds. (Brindha et al., 1981).
Reducing sugar
Reducing sugar is present in the chloroform extracts of all three parts powders, whereas it present only in acetone extract of bark powder. (Brindha et al., 1981).
Saponins
Saponins are present in the benzene and aqueous extracts of both bark and root powders, while they occurs only in the chloroform extract of bark powder. (Brindha et al., 1981).
Steroids
Steroids are present in the benzene and chloroform extracts of bark powder, whereas ether extract of root contains steroids. (Brindha et al., 1981).
Tannins
Acetone and ethanol extracts of both bark and root powders contain tannins, while they present only in the benzene extract of root powder. (Brindha et al., 1981).
The plant contains bioactive compounds such as flavonoids, phenols, reducing sugars, saponins, steroids and tannins. Flavonoids are extracted through all solvents in the plant and few compounds are extracted only through specific solvents. However, root contains more chemical compounds, compared to bark and leaf.
However, aqueous extracts of all the three parts powders contain tannins. In general, organic solvent extracts of Odina wodier showed presence of several secondary metabolites except alkaloids. Similarly, the leaf ethanolic extract (LEEX) and root ethanolic extract (REEX) of Landolphia owariensis indicated the presence of flavonoids, saponins and tannins in both the leaf and root extracts with cyanogenic glycosides present in the root extract only (Nwaogu, et al., 2008).
The extracts of Plectranthus glandulosus by using hexane, ethyl acetate and ethanol as solvents were screened for secondary metabolites and found that the presence of tannins, reducing sugars, saponins, flavonoids and steroids. Steroids were present in hexane and ethyl acetate extract but absent in ethanol extract (Egwaikhide and Gimba, 2007).
The phytochemical analysis of the crude extracts of 12 traditionally used Indian medicinal plants indicated that the presence of major phytocompounds, including phenolics, alkaloids, flavonoids, and tannins, which may have been responsible for the observed antioxidant activity (Aqil et al., 2006).
The phytochemical screening of Parkia biglobosa showed that the extracts of the stem bark were rich in sterols, triterpenes, tannins, saponosides, anthocyanidins, flavonoids, and reducing compounds. The leaves extracts were rich in tannins, anthocyanins, flavones and reducing compounds (Millogo-Kone et al., 2006).
Cheriti et al. (2004) have investigated the phytochemical screening of some 25 commonly occurring medicinal plants, which exhibited the presence of flavonoids, tannins, alkaloids, saponins and sterols. The extract of Swertia (Gentianaceae) showed the presence of many bioactive components such as irioids, xanthones, flavonoids and polyphenolic components (Hajimehdipoor et al., 2004).
Antibacterial Activity of crude extracts
Leaf
Acetone extract of leaf of Odina wodier showed significantly greater inhibitory activity against Klebsiella pneumoniae (0.43cm), compared to that of Bacillus subtilis (0.3cm) and Streptococcus faecalis (0.3cm). (Fig.1-6:Fig.A1-A5) Similarly, ethanol extract of leaf showed maximum inhibitory activity against Streptococcus faecalis (0.43cm) followed by Klebsiella pneumoniae (0.4cm) and Escherichia coli (0.38cm). Benzene extract of leaf showed maximum inhibitory activity against Escherichia coli (0.28cm) followed by Proteus vulgaris (0.23cm). Pseudomonas aeruginosa (0.3cm) was inhibited greatly by the chloroform extract of leaf followed by Staphylococcus aureus (0.23cm), Bacillus subtilis (0.23cm) and Escherichia coli (0.23cm). Micrococcus luteus (0.25cm) and Streptococcus faecalis (0.25cm) were more sensitive to ether extract of leaf than other Pathogenic organisms studied. An aqueous extract of leaf showed maximum inhibitory action against Pseudomonas aeruginosa (0.3cm) followed by Escherichia coli (0.23cm) and Staphylococcus aureus (0.23cm). However, Acetone, ethanol and ether extract of leaf did not showed any significant inhibitory activity against Pseudomonas aeruginosa and Staphylococcus aureus.
Bark
Acetone extract of bark showed greater inhibitory activity against Proteus vulgaris (0.38cm) than that of other pathogenic organisms, while benzene extract of bark showed maximum inhibitory action against Streptococcus faecalis (0.3cm) than that of other pathogenic organisms. (Fig.1-6:Fig.B1-B5) Chloroform extract of bark showed maximum inhibitory action against Bacillus subtilis (0.23cm) and Micrococcus luteus (0.23cm) followed by Escherichi coli (0.2cm), Proteus vulgaris (0.2cm) and Staphylococcus aureus (0.2cm), whereas, ethanol extract of bark showed maximum inhibitory activity against Staphylococcus aureus (0.33cm) followed the Klebsiella pneumoniae (0.23cm). Ether extract of bark showed greater inhibitory activity against Pseudomonas aeruginosa (0.2cm) followed by Streptococcus faecalis (0.15cm) and Bacillus subtilis (0.15cm). However, an aqueous extract showed maximum inhibitory activity against Escherichia coli (0.25cm) followed by Proteus vulgaris (0.2cm).
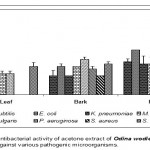 |
Figure 1
|
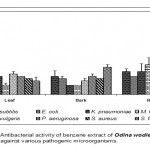 |
Figure 2
|
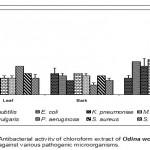 |
Figure 3
|
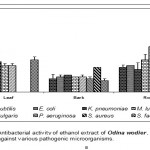 |
Figure 4
|
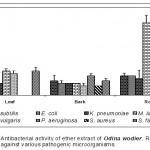 |
Figure 5
|
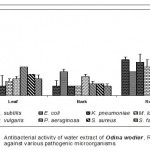 |
Figure 6
|
Root
Acetone extract of root showed significantly greater inhibitory action against Proteus vulgaris (0.78cm) followed by Staphylococcus aureus (0.65cm), compared to other pathogenic organisms (Fig.1-6:Fig.C1-C5) Similarly, Proteus vulgaris (0.43cm) showed more susceptibility to benzene and chloroform extracts of root. Ethanolic extract of root showed significantly greater inhibitory action against Staphylococcus aureus (1cm) compared to other pathogenic organisms studied. Ether extract of root showed significantly greater inhibitory action against Micrococcus luteus (0.75cm) than that of other pathogenic organisms. Aqueous extract showed maximum inhibitory action against Bacillus subtilis (0.38cm), Proteus vulgaris (0.38cm) and Streptococcus faecalis (0.38cm).
The medicinal plant of Odina wodier extracts have great potential to control the pathogenic organism studied. Root extracts of plant showed a maximum inhibitory activity against the pathogenic organisms studied. Acetone and ethanol extracts of Odina wodier, showed the greater inhibitory activity to that of all other extracts (Benzene, Chloroform, Ether and Water). However, ether extracts of Odina wodier showed the least inhibitory activity against pathogenic organisms studied.
Acetone and ethanolic extracts of Odina wodier showed maximum inhibitory activity against Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae and Streptococcus faecalis. Ether and Benzene extracts of root showed maximum inhibitory activity against Staphylococcus aureus and Proteus vulgaris.
Having in general, results of the present study reveal that the root extracts in organic solvents are the best medicinal properties in terms of inhibitory action against pathogens than that of the other parts. Similarly, the crude leaf extract of Stevia rebaudiana in different solvent system proved to be very effective antimicrobial agent. The methanolic extract was the most effective against all the bacteria. The activity was similar to the antibiotic tested. Among the bacterial species maximum inhibition was found in S. aureus, followed Streptococcus mutans, B. subtilis and E. coli. The crude leaf chloroform extract of S. rebaudiana leaves was also found to be effective than aqueous extract for all the bacteria tested. Maximum inhibition on comparative scale was found in S. aureus, S. mutans, B. subtilis followed by E. coli on the basis of the zone of inhibition (MIC). The activity of the extract was found to be compared with 1% concentration of antimicrobial used (streptomycin, nystatin).The aqueous extract was found not effective to all the microorganisms tested. When the growth of the micro-organisms against the solvents (without leaf extract of S. rebaudiana) was evaluated, no marked inhibition of growth was observed. On various dilution of the crude leaf extracts, similar results confirmed. It was also observed that on dilutions the inhibition in some cases was more. The aqueous extract was found to be non-effective for all bacteria. The chloroform extract on dilution showed mild inhibition against the bacterial growth. Maximum inhibition zone was shown by S. aureus, S. mutans, B. subtilis followed by E. coli on the basis of the zone of inhibition. This is a clear indication that the solvent system plays an important role in the solubility of the antimicrobial substance and also affects the antimicrobial activity (Debnath, 2008). However, ethanolic, ether and acetone extracts of root showed significantly greater inhibitory activity than that of other extracts.
The ethanol extract of the aerial parts of Sida acuta showed significant inhibitory activity against standard strains and clinical isolates of Staphylococcus aureus, clinical isolates of Bacillus subtilis and Streptococcus faecalis. Neither the concentrated extract nor its dilutions inhibited Escherichia coli, Klebsiella pneumonia and Pseudomonas aeruginosa. The results demonstrate that the crude extract of the aerial parts of Sida acuta has a narrow spectrum of activity and suggest that it may be useful in the treatment of infections caused by Gram positive aerobic bacteria (Oboh et al., 2007).
The crude extracts of Dorema ammoniacum, Sphaeranthus indicus, Dracaena cinnabari, Mallotus philippinensis, Jatropha gossypifolia, Aristolochia indica, Lantana camara, Nardostachys jatamansi, Randia dumetorum and Cassia fistula exhibited significant antimicrobial activity and properties that support folkloric use in the treatment of some diseases as broad-spectrum antimicrobial agents (Kumar et al., 2006).
The highest antibacterial action of the acetone extract of Combretum molle was against the Gram negative organisms Escherichia coli. The activity of the extract against these bacteria was comparable to that of ciprofloxacin when assessed by the disc diffusion technique (Asres et al., 2006).
The difference in the inhibitory activity could be due to several factors, such as chemical nature of components, differential extraction, partition and quantity of active ingredients present in the different extracts.
In comparison with the three different parts of the plant (Odina wodier) studied, (leaf, bark and root), root is better in terms of antibacterial action compared to the bark and leaf. The present study conclude that the plant extracts of root have a great potential as antimicrobial compounds against pathogenic micro-organisms and that they can be used in the treatment of infectious diseases caused by them. Root extracts show maximum antibacterial activity and so this part of the plant can be used to discover bioactive natural products that may serve as leads for the development of new pharmaceuticals that address hither to unmet therapeutic needs.
References
- Aqil, F., Ahmed, L. and Mehood, Z., Antioxidant and free radiant properties of twelve traditionally used Indian medicinal plants. Faculty of Agricultural Sciences, 30: 177-183 (2006).
- Ahmad, L. and Beg, A.Z., Antimicrobial and phytochemistry studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology, 74: 113-123 (2001).
- Akinpelu, D.A. and Onakoya, T.M., Antimicrobial activities of medicinal plants used in folklore remedies in South – Western. African Journal of Biotechnology, 5: 1078-1081 (2006).
- Asres, K., Muzumnder, A. and Bucar, F., Antibacterial and antifungal activities of extracts of Combretum molle. Ethiop med j, 44(3): 269-277 (2006).
- Brindha, P., Sasikala, B. and Purushothaman, K.K., Pharmacognostic studies in merugan kizhangu. AMEBR, 3: 84-86 (1981).
- Cheriti, A., Belboukhari, N. and Hacini, S., Ethnopharmacological survey and phytochemical screening of some medicinal plants of Algerian Sahara. Journal of Pharmaceutical Research, 2: 51(2004).
- Egwaikhide, P.A. and Gimba, C.E., Analysis of the phytochemical content and antimicrobial activity of Plectranthus glandulosis whole plant. Middle-East Journal of Scientific Research, 2(3-4): 135-138(2007).
- Hajimehdipoor. H., Mariotte, A., Franca, M.D., Amanzadeh, Y., Sadat Ebrahimi, S.E. and Khansair, M .G., Phytochemical study of Swertia longifolia. Iranian Journal of Pharmaceutical Research, 2: 15-16(2004).
- Kumar, V.P., Chauhan, N.S., Padh, H. and Rajini, M.B., Search for antibacterial and antifungal agents from selected Indian medicinal plants. Journal of Ethnopharmacology, 107(2): 182-8 (2006).
- Langfield, R.D., Scarano, F.J., Heitzman, M.E., Kondo, M., Hammond, G.B. and Neto, C.C., Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. Journal of Ethnopharmacology, 94: 279-281(2004).
- Latha, M., Ramkumar, K.M., Pari, L., Damodaran, P.N., Rajeshkannan, V. and Suresh, T., Phytochemical and antimicrobial study of an antidiabetic plant: Scoparia dulcis L. J. Med Food, 9(3) 391-394 (2006).
- Millogo – Kone, H., Guissoe, P.I., Nacoulma, I. and Traore, A.S., Study of the antibacterial activity of the stem, bark and leaf extracts of Parkia biglobosa. African Journal of Traditional, Complementary and Alternative Medicines, 3: 2 (2006).
- Nadkarni, K.M ., Indian material medica. Popular Prakashan Pvt. Ltd., 1: 867-868 (1998).
- Ncube, N.S., Afolayan, A.J. and Okoh, A.I., Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African Journal of Biotechnology, 7(2): 1797-1806 (2008).
- Nwaogu, L.A., Alisi, C.S., Igwe, C.V. and Ujowundu, C.O., A comparative study of the antimicrobial properties of the ethanolic extracts of Landolphia owariensis leaf and root. African Journal of Biotechnology, 7(4): 368-372 (2008).
- Oboh, I.E., Akerele, J.O. and Obasuyi, O., Antimicrobial activity of the ethanol extract of the aerial parts of Sida acutaf, (Malvaceae). Tropical Journal of pharmaceutical Research, 6(4): 809-813 (2007).
- Parekh, J. and Chand, S., In vitro Antimicrobial activity of extracts of Launaea procumbens. Roxb. (Labiateae), Vitis vinifera L. (Vitaceae). African Journal of Biomedical Research, 9: 89-93 (2006).
- Raghavendra, M.P., Satish, S. and Rveesha, A., Phytochemical analysis and antibacterial activity of Oxalis corniculata; a known medicinal plant. Journal of Science, 1: 72-78 (2006).
- Warrier, P.K., Nambiar, V.P.K. and Ramnkutty, C., Indian medicinal plants – a compendium of 500 species. Orient Longman Ltd., 3: 297-299 (1995).
- Woldemichael, G.M., Wachter, G., Singh, M.P., Maiese, W.M. and Timmerman, B.N., Antibacterial activity of folk medicinal plants. Journal of Natural Products, 66: 242-246 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.





