How to Cite | Publication History | PlumX Article Matrix
Esther Kibuka-Sebitosi
University of South Africa, Institute for African Renaissance Studies, 287 Skinner Street, P.O. Box 392, UNISA 0003, Pretoria South Africa.
ABSTRACT: The feeding behaviour of wild caught G. pallidipes and laboratory reared G. m. morsitans was investigated following topical application of deltamethrin and natural pyrethrum extract in acetone. Flies were weighed before and after every blood meal (on rabbits) and maintained at 250C and 65-70% relative humidity. Females with a 3rd instar larva were separated, observed until larviposition and larvae watched for pupation, until death. Results showed that both G. m. morsitans and G. pallidipes were more susceptible to deltamethrin compared to pyrethrum extract. Flied fed in groups showed varied feeding behaviour while those feed individually revealed a 4-day feeding cycle. The treated females demonstrated a 7-day feeding cycle that was characterized by frequent but small blood meals. Female. G. Pallidipes treated with 0.0001-0.01ng deltamethrin showed a peak of 35 mg blood meal. These results showed that insecticides disrupted feeding cycles of treated flies. Failure of flies to feed means a reduction in the reserves and a reduction in the survival with implications on transmission of trypanosomes. Flies that recovered were probably due to abortions; inert storage of insecticides in the fat body; and partitioning of the insecticides into the uterine milk gland during pregnancy. It appears that sublethal doses of insecticides have a role to play in the feeding behaviour, subsequent pupal weights, progeny and tsetse fly population and should be taken into consideration when designing control strategies. All these factors are discussed in relation to the tsetse fly population dynamics; control and transmission challenges. The potential threat to other insects that occupy similar ecosystems with tsetse flies is highlighted calling for more sustainable and environmentally friendly control methods.
KEYWORDS: Tsetse flies; insecticides; Deltamethrin; Pyrethrum extract; Feeding behaviour
Download this article as:| Copy the following to cite this article: Sebitosi E. K. Sublethal Effects of Insecticides on Feeding Behaviour of Testse Flies Glossina Pallidipes Austen and Glossina Morsitans Morsitans (Westwood) (Diptera: Glossinidae). Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Sebitosi E. K. Sublethal Effects of Insecticides on Feeding Behaviour of Testse Flies Glossina Pallidipes Austen and Glossina Morsitans Morsitans (Westwood) (Diptera: Glossinidae). Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9175 |
Introduction
Tsetse flies are blood-sucking dipteran insects belonging to the genus Glossina. They are confined to tropical Africa (Buxton, 1955; Nash, 1969). Tsetse present a significant health hazard, being vectors of Trypanosomes that cause human Trypanosomiasis (sleeping sickness) and animal Trypanosomiasis (nagana). According to the World Health Organization (WHO, 2001),60 million people are at risk of contracting the disease in 37 countries which is approximately 40% of Africa (8.5 million km2).Amidst the epidemic, new infections and mortality are increasing. The disease burden is over 2.05 million with the breakdown in surveillance, conflict, civil strive and war particularly in the Central and Horn of African region covering Democratic Republic of Congo and the Sudan, among other countries; the prevalence is difficult to estimate under these circumstances (Moore et al 1999; Ekwanzala et al 1996; Van Hove, 1997; Barrett, 1999; Smith et al., 1998; WHO, 2001; Allsopp, 2001).
In view of the magnitude of this problem, great efforts in the past have been directed in the past towards controlling tsetse flies by a variety of methods, including game elimination (removing their food supply) and bush clearing (destroying their preferred habitats) (Nash, 1940).Tsetse flies are adapted to vast habitants ranging from open savannah in East Africa to Tropical rain forests in West Africa. This in turn has influenced the control mechanisms as ecological, political and neo-colonial aspects emerge in the control strategies; with approaches in West Africa focusing on disease control while vector control is emphasized in East and Southern Africa.
Following the discovery of the insecticidal properties of DDT in 1944, the control of tsetse flies, like many other insect pest problems, became increasingly dependent upon chemicals. Recently, alternative methods have been tried with littlesuccess and still others are being developed. These include the sterile male techniques, use of traps, screens and targets, use of new potent odour attractants and the use of tsetse pathogens to control tsetse populations, (Allsopp, 1988; Carpels and Greathead, 1989; Dagnogo et al., 1988; Sebitosi et al., 1998; Jordan and Trewen, 1974; Kangwagye et al., 1988; Kupper, 1988; Mawuena, 1988; Opiyo et al., 1988; Vale et al., 1988).
The tsetse control increased with the development of the bait techniques, in addition to the targets and screens. These later emerged into live baits of cattle on which insecticides have been poured with some success. With the availability of synthetic pyrethroids of low mammalian toxicity and high efficacy, tsetse control using insecticides became popular (Vale et al., 1988; Shereni, 1997). However, there is evidence that re-invasion occurs in such areas (Davies, 1981). The environmental effects of non-target organisms and the environment risk continue to be a concern (Grant, 2001). Can the effects of sublethal doses of insecticides ward off, at least for some time before the next spray or intervention, such re-invasions?
When insecticides are sprayed in the field, with the view to eradicating insect pests, it is inevitable that a certain proportion of the target population will be exposed to doses less than the threshold concentration. The effects of sublethal doses of insecticides on tsetse flies are not fully known. Given that one thirds of Africa’s population live under tsetse fly infested areas, eliminating tsetse flies is fundamental. The environmental issues surrounding the alleviation of Trypanosomiasis are among the most debatable around the world. The concern is land degradation, extensive use of insecticides and intensification of livestock production (De Haan et al., 1997; Grant, 2001). In the wake of globalization and economic crush rising food costs, tsetse fly elimination or control seem to be viable options.
In Glossina the reproductive process is particularly vulnerable to interferences due to its innate complexity and the low fecundity of tsetse flies. For example, it has been shown that reduction in the frequency of blood meals will adversely affect larval development by inducing abortion, or producing under-weight and weak larvae with less chances of surviving the harsh environment (Saunders, 1962).
The need for more laboratory research relevant to the control of tsetse was stressed by many authors. At present, there is much interest in developing new methods of insect pest control. Alternatives are being sought to broad spectrum organic insecticides, which would disrupt the physical processes within the insects. Such compounds with different modes of action and varying rates of persistence within the insect are likely to differ in character and extent of their effects. Knowledge of the response of tsetse to low doses of insecticides would improve assessments of the relative efficacy of insecticides against them in the field. Such information is useful in a number of ways, including the understanding of the tsetse fly population dynamics in frequently sprayed areas. In this connection, the following questions become pertinent:
Is the population suppressed further due to sublethal effects, after the spray?
Are the flies receiving sublethal doses feeding normally after the spray?
What are the long term implications of sublethal effects on feeding and extrapolation to subsequent transmission?
This paper illustrates the effect of sublethal doses of pyrethrum extract and deltamethrin on feeding asurvival of G. pallidipes and G. m. morsitans andthe overall tsetse fly managementstrategies.
Materials and Methods
Tsetse Flies
Mated female tsetse flies of two species Glossina pallidipes and Glossina morsitans morsitans were used in these experiments.Specimens of G.m.morsitans were obtained from a colony originating from Langford, Bristol, U.K, and maintained at the International Center of Insect Physiology and Ecology. Teneral females were fed for four days and then allowed to mate with sexually mature males (at least 7 days old) for a period of 24 hours by introducing the males in a 1:1 ratio to the females. Mated females were kept in an insectary maintained at 25ìC and 65-70% relative humidity and fed daily on rabbits.
Glossinapallidipes adults were collected from Nguruman near Magadi town, 70 Km southwest of Nairobi where the species inhabits a wooded bushland and open grass alluvial plains of semi-arid land together with G.longipennis. Dominant trees are Acacia albida (Del), Ficus capensis (Thumb), and Acacia tortillus (Forsk). The abundant grasses are Sporobolis consimulis (Foesk) and Cynodon dactylon (L).
Common game species which provide the tsetse with readily available bloodmeal hosts include the bushbuck (Tragelaphus seriptus Pallas), buffalo (Syncerus caffer Sparrman), impala (Aepyceros melampus Lichtenstein), dikdik (Rhynotrogus kirki Thomas), giraffe (Giraffa camelopardis (L)), zebra (Equus burchelli L), and hyena (Crocuta crocuta Erxleben). The area is inhabited by the Masai people who herd cattle and goats in the plains.
Tsetse flies were collected using, biconical traps (Challier and Lavéissière, 1973) baited with acetone and cow urine to increase the catches. The flies were transferred to polyvinyl chloride cages (PVC) which were made of Terylene netting with one end fitted with a cork. While still in the field, the flies were sexed and the females given a blood meal by placing the cage of flies on the ears of rabbits restrained in wooden boxes. The cages containing the flies were tightly attached to the ears with rubber bands and covered with black cotton cloth during the feeding period.
Proper packaging for safe transportation was necessary to reduce the risk of abortions and mortalities. To this end, the PVC cages were lined with soft tissue blotting paper to help provide moisture over the entire cage surface and to prevent excreta from attaching on to the wings. The cages in turn, were kept moist by wet cotton wool and covered on the outside with damp black cotton cloth to minimize flight activity in the cage to conserve fly energy. The cages were packed in paper cartons lined with damp black cloth to conserve moisture during transportation to Nairobi.
In the laboratory, mass rearing techniques normally used to maintain laboratory colonies of tsetse were employed to rear the two species (Rogers and Kenyanjuyi, 1971). Both species were kept in an insectary maintained at 250C, 65-70% relative humidity, and under a 12 hr light/dark cycle with lights off at 18.00 hrs.Female flies from the field were held for a period of one week before treatment to screen out mortalities due to handling and to acclimatize them to the blood meal from rabbits. The same duration allowed the flies to getused to standard laboratory conditions, such that any response during experiments would be attributed to treatment effects.
To standardize the material further, teneral flies were excluded from the samples. The pregnant females, which were recognized by the abdominal appearance (Saunders and Dodd, 1972) due to a late second or third-instar larva in utero were also excluded. Thus, all treated females were, therefore, as homogeneous as possible with respect to their pregnancy state. They were presumably in early pregnancy, being the flies which would be in a hunger stage and come to the traps in the field (Vale, 1974).
Rearing
To ensure that flies were available for experiments, rearing was necessary using standard PVC cages of 18 cm x 4 cm x 5 cm. Both species were maintained under similar laboratory conditions (250 C and 65-70% relative humidity). Feeding took place daily to eliminate any effects due to starvation. The feeding time allowed per cage was 10 minutes.
All flies carrying 3 rd instar larvae were separated from the mass-rearing PVC cages and introduced singly into polystyrene observation tubes (6.5 x 8 cm) in order to synchronize the larviposition and the next pregnancy cycle. The tubes were then placed on a tray covered with wire mesh.
In addition, each pupa produced was maintained singly in the plastic tube in the insectary, together with labels indicating its origin. This allowed a follow-up of the flies individually both before and after experimental manipulations.
A fly from each of the two species was removed from the PVC cages using a glass tube, fitted with terylene netting on one end. To prevent the fly from escaping, the opening on the PVC cage was firmly covered with a sponge fitted on the cage by rubber bands. The female was carefully blown by mouth into the palm of the hand. Taking care not to squash it, the fly was held by the thorax. All flies were hand-held while treating without prior anesthesia, to avoid any stress due to the anesthetics.
Topical Insecticide Application
A micro litre (µl) quantity of insecticide solution was applied to the dorsal thorax (mesonotum) of the individual flies, using the microapplicator fitted with a 1 ml syringe and a 27 gauge needle. They were returned to clean tubes, offered a bloodmeal 24 hrs later, and maintained as described above.
Contact Insecticide Exposure
For contact treatments, the flies were held for 30 seconds on surfaces treated with the insecticide. These surfaces were glass slides and blue cotton cloth pieces cut to measure 7.5 x 2.5 cm rectangular and 4.3 x 4.3 cm square respectively. The cotton blue cloth was selected because it is used in the construction of biconical traps for tsetse control.
A known volume (0.5 µl) of insecticide solution was evenly spread on the glass or cloth surface using a hand held 200µl microapplicator fitted with the proper nozzle, which was adjusted to ensure even spreading of the drops in equal sizes. The spreading process started in one corner and spread in a straight line, to cover the whole surface. The solvent (acetone) was allowed to evaporate for 15 min, leaving the active ingredients on the surfaces.
Female flies, which had been fed 24 hr previously, were removed from the cages as described above. Preliminary experiments involved exposing individual females to the surfaces for varying periods of time from 10 seconds to 1 minute were carried out. The 30 second duration gave consistent survival and hence was chosen as the exposure period. The control flies were exposed to acetone treated surfaces alone. Ten flies were exposed, by hand-holding, in each of three replicates then transferred to the single plastic tubes and maintained in the insectary as described above.
Mortality counts were made 48hr after treatment. The results were analyzed using probits, and susceptibility curves drawn graphically (Lichtfield and Wilcoxon, 1949; WHO, 1970; WHO, 1975).Sublethal doses comprised those concentrations that killed a proportion of the tsetse flies within a specified period of time, which was 48 hours in this case. The survivors of LD10, LD30 and LD50 ng were selected and observed for the existence of “sublethal effects”, in terms of feeding behaviour and reproductive performance (Baldry, 1964; Kwan and Gatehouse, 1978).
Blood meal size
Flies were each weighed immediately before and after the blood meal, before the fly excreted. The blood meal per day was calculated and compared with that taken by the acetone treated females. The blood meal were followed for at least five pregnancy cycles or until death.
Maintenance of flies and observation after treatment
After treatment with insecticides, the flies were maintained individually in tubes made from clear polystyrene (4.5 cm diameter and 9 cm height) at 250C and 65-70% relative humidity. The top of each tube was covered with Terylene netting held in place by rubber bands and permanently stuck with adhesive. The tubes were left for 48 hr to allow the glue to dry before flies were introduced. This prevented any mortality due to the fumes of the glue. At the top of each tube, a small hole was cut to allow the introduction of the fly which was later kept sealed with a self-adhesive label, to prevent the fly from escaping. The following observations were made:
Knock down effects, in terms of time taken for the flies to recover, from the initial knockdown insecticide characteristics.
Post treatment survival, as number of days survived.
Feeding behaviour of the survivors, in terms of quantity taken in and frequency of feeding in a pregnancy cycle.
Results
Post-treatment survival of G.pallidipes and G.m.morsitans females following treatment with deltamethrin and pyrethrum extract
The susceptibility of G. pallidipes and G. m. morsitans to deltamethrin and pyrethrum extract 48 hr after topical application (Figure 1), or exposure to insecticide treated glass or cloth given in Fig. 1A and B showed that G. pallidipes was more susceptible to deltamethrin compared to pyrethrum extract (Fig. 1A). On the other hand, the mortality after exposure to insecticide treated glass and cloth was erratic (Fig. 1B and C) for both species.
Furthermore, analysis of variance showed that the three methods of insecticide application were significantly different (F = 3.54, P < 0.03, n = 95) from each other when the acetone controls were compared for G. pallidipes. The relative frequencies of survival by the methods (Fig.1 A,B&C) for G. pallidipes (A) and G. m. morsitans (B) showed that the topical method of application gave the best survival. Glass and cloth (Fig. 1 C), on the other hand, gave erratic survivals and seem to have reduced it altogether. This led to the choice of topical application as the best method of sublethal dose application. The rest of the data in the subsequent chapters, therefore, is based on results obtained from topical application.
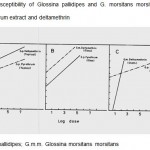 |
Figurre 1: Susceptibility of Glossina pallidipes and G. morsitans morsitans to natural pyrethrum extract and deltamethrin.
|
Pyrethrum extract had no significant effect on the survival of G. pallidipes females (r = -0.19, P> 0.1 – 0.5, n = 297; Table 2). The data on deltamethrin was transformed into Log (concentrations) in order to spread out the very low concentrations. Similarly, no significant effect (r = -0.0027, P>0.1, n = 90) of deltamethrin on G. pallidipes was observed on survival(Table 2). However, a significant effect of pyrethrum extract (r = -0.33, P<0.001, n= 66) on the survival of G. m. morsitans was observed (Table 2). Deltamethrin also had a significant effect (r = -0.55, P<0.001, n = 48) on G.m. morsitans post treatment survival, following topical application (Table 2).
The frequency distribution of the survival of G. pallidipes and G.m. morsitans females in days for all the treatments is given in Fig. 2. The number of living flies decreased as the period since treatment increased, in both species. Relatively, a bigger proportion (0.31) of G. pallidipes survived for 20 days and only 0.06 lived up to 50 days following insecticides treatment. G. m. morsitans, on the other hand, had more (0.44) surviving up to 10 days and close to 0.51 living for 50 days (Fig. 2). The G. m. morsitans females having been obtained from a laboratory colony had uniform ages. However, G. pallidipes from the field had variable ages.
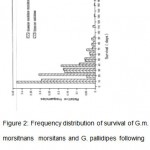 |
Figure 2: Frequency distribution of survival of G.m.morsitnans morsitans and G. pallidipes following insecticide treatment (Data pooled).
|
Blood meal size of deltamethrin and pyrethrum treated G. pallidipes and G. m. morsitans
Table 3 shows the mean blood meal taken per day for the two species during the first ten days after insecticide application. The mean blood meal per day taken by G. pallidipes during the first ten days after pyrethrum extract treatment ranged between 13.10 mg and 15.04 mg (15.15 mg for acetone controls). G. m. morsitans, on the hand, had blood meals ranging from 14.26 mg to 16.99 mg (14.41 mg for acetone controls). No significant effect due to pyrethrum extract on either species on the mean blood meal size per day was apparent (Fig. 3). It is also clear from Fig. 4 that there was no significant effect of deltamethrin on the mean blood meal weights for G. pallidipes, therefore, no further analysis was necessary. Fig. 3 showed a relatively higher blood meal intake for females treated with 5 ng /ml pyrethrum extract. This, however, should not be taken to mean an increase in blood meal intake following a relatively high dose treatment. Rather, it was observed that high doses caused the females to abort. Following abortions, the females took large blood meals.
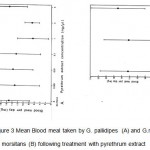 |
Figure 3: Mean Blood meal taken by G. pallidipes (A) and G.m.morsitans(B) following treatment with pyrethrum extract.
|
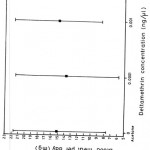 |
Figure 4: Blood meal weight taken by G. pallidipes following treatment with deltamethrin.
|
Blood meal size and survival
The correlation between the size of the blood meal ingested by the G. pallidipes females treated with deltamethrin and the number of days they survived (following treatment) was significant (r = 0.67, P<0.001, n = 26). However, no significant effects of deltamethrin concentrations on either survival (r = 0.11, P> 0.1, n = 90 ) or blood meal weight (r = 0.042, P> 0.05 – 0.001, n = 26) were separately detected.
Discussion
Insecticides sprayed in the field, and not reaching their target insects in sufficient quantities to kill them have an important role to play in the physiology of these insects. The present studies have shown that sublethal doses of insecticides have, significant effects on the survival of G.m.morsitans and feeding behaviour of the two species.
The importance of these effects lies in the proportion of the population that gets affected. There is reason to believe that this proportion is quite substantial, because a large percentage of females rest under canopy. Therefore, when blanket spray of insecticide is carried out, many flies do not get sufficient doses to get killed (Leroux and Patt, 1968; Moisier, 1912; MacDonald, 1960; Carnevale & dam,1971; Challier, 1973; Baldry, 1980). The use of helicopters for tsetse control was discussed in detail by Baldry et al. (1978), Molyneux et al. (1978) and Van Wettere et al. (1978). It was concluded that insufficient droplets were deposited to effect control of G.tachinoides (Lee et al., 1980) and that the residual effect would largely depend upon the flies vertical movements as described by MacLennan & Cook (1972), specifically for G.m.morsitans. Other authors (Johnstone et al., 1974) also questioned the penetration of residual helicopter application. The use of helicopters to apply dieldrin into dense thickets in Kenyas’ Lambwe valley (Leroux&Patt, 1968) revealed similar problems. This would suggest that mortality counts made at 24 and 48 hours in order to determine the efficacy of the insecticide, may be misleading and perhaps should be revised to give a true picture of mortality due to insecticide treatment.
Results have also demonstrated no significant effect of the two insecticides on the feeding of both G. pallidipes and G. m. morsitans. Exposure to dieldrin at low doses has been reported to inhibit feeding in female Aedes aegypti L. (Duncan, 1963). However, there was no indication of any effect on the incidence of feeding by G. m. morsitans 48 hr after exposure to dieldrin, endosulphan or permethrin (Kwan and Gatehouse, 1978). They suggested that the minor effects of the solvent (butanon) required further investigation, although they never resulted in a reduction of in the total blood uptake during the first 7 days following the post-treatment meal.
The relationship between the blood meal and survival showed a significant positive correlation between the two. This indicated that flies that took large blood meals also survived longer implying potential vectorial capacity. The small but frequent blood meals also imply further transmission possibilities if hosts are available.
Sublethal doses did not affect the blood meal size significantly. Against this background, it is proper to conclude that sublethal doses of the two insecticides did not reduce the size of the blood meal and subsequently the flies receiving such doses can feed normally. The behaviour of the flies however, showed a trend of feeding small but frequent blood meals. This frequency of feeding may have implications in the transmission of the trypanosomes. Studies in this area would be needed to clarify this observation.
Results from the survival have shown that both deltamethrin and pyrethrum extract reduced the survival of G.m.morsitans but not of G.pallidipes. This difference may be genetic in origin. Nevertheless it implies that sublethal doses of these insecticides have an adverse effect on the survival of the flies. If such flies do not survive well, then these low doses do make a significant contribution to the fly mortality after spray application. These factors contribute to the fly population stress.
The question that arises is at what stage and what proportion of the population could they be effective?Irregular feeding was the main cause of abortions, an observation similar to that of Mellanby (1937). The same author noted that egg maturation and “milk” production are dependent upon the nutrients made available from the blood meal of the female, and that the pupal weight could be directly connected with the total amount of blood taken during the pregnancy cycle.Burnett (1963), Irving (1968), Kwan et al. (1982) and Riordan (1987) all reported an increase in tolerance of pregnant flies to insecticide treatment. Burnett (1963) suggested that tolerance raised for a low dose of fenthion was due to diffusion of insecticide into the in utero embryo. Similar diversion of DDT into the in utero larva was termed “inert storage” by Irving (1968), and Kwan et al (1982) suggested that partitioning of endosulphan into lipids in the maternal fat body during early pregnancy and in uterine milk during late pregnancy, explained increasing in tolerance through each pregnancy cycle. The effects of the deltamethrin causing abortions are dealt with in another publication and have been it was suggested by Buxton (1955) that this could be due to undernourishment of individual flies.
It is clear that low-dose insecticide effects on reproduction do cause a significant contribution to the overall mortality of the tsetse fly population. When insecticides are applied therefore, those flies that get sublethal doses, although they are not affected in terms of reducing their feeding behaviour, they are affected through induction of abortions, reproductive abnormalities inside their ovaries, and pupal development retardation. The effects on beneficial insects such as butterflies and bees, however, should not be underestimated, even at low dose exposure.
It has been demonstrated that sublethal doses of pyrethrum extract and deltamethrin affect tsetse survival. Further studies are recommended using radio labeled deltamethrin in vivo , which would show where the sites of action are. Also, the effect of these insecticides on the F2 and F3 should be investigated to establish the long term effects on the progeny, if any.
Acknowledgements
I wish to thank Dr. M.F.B. Chaudhury for his support and teaching me about Tsetse fly physiology. I acknowledge Dr. Edward Vanden Berghe for assistance in statistical analysis and Dr. Dransfield & Kyorku, for field population dynamics and trapping of tsetse flies. Technical support from Mr. D. Iuyu, Kasuti and P. Lisamula is appreciated. I also wish to acknowledge institutional support of the International Centre for Insect Physiology (ICIPE), Nairobi, Kenya.
References
- BALDRY, D.A.T., 1980. Local distribution and ecology of Glossina palpalis and tachinoides in forest foci of West African human trypanosomiasis, with special reference to associations between peri-domestic tsetse and their hosts.Insect.Sci.Applic. 1, 85-93.
- Baldry, D.A.T., Molyneux, D.H., Van Wettere, P., 1978. The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachinoides in West Africa. Evaluation of decamethrin applied as a spray. Pest.Artic.and News Summ. 24, 447-454.
- Barret, M.,1999. The fall and rise of sleeping sickness, Lancet 353, 1113-1114.
- BURNETT, G.F., 1963. The susceptibility of tsetse flies to topical applications of WHO/Vector control/37.
- BUXTON, P.A., 1955. The natural history of tsetse flies.London school of Hygiene and Tropical Medicine, Memoire No.10. H.K. Lewis, London.
- CARNEVALE, P. , Adam, J.P.,1971. Contributions to the biological study of Glossinapalpalispalpalis R-D in the popular Republic of Congo.pp.207-211 in Int. Sci. Council Trypanosomiasis. Res. Thirteenth Meeting, Lagos, Nigeria.328 pp. Niamey,Niger.OAU Publications Bureau, Maison de l’Afrique.
- Carpels, G.I., Greathead, D.J., 1989. A record of Exhyalanthrax abruptus (Loew) (Diptera, Bombyliidae), a tsetse parasitoid from the Luangwa Valley, Eastern Province, Zambia.Annales de la Societe belge de Me’decine tropicale. 69 (2), 157-159.
- CHALLIER, A.,1973. Ecologie de Glossina palpalis gambiensis Vanderplank, 1949 (Diptera: Muscidae) en Savane d’Afrique occidentale 274 pp. Paris France ORSTOM (Memoires ORSTOM no.64).
- Dagnogo, M., Eouzan, J.P. , Lohuirignon, K.,1988. Le piegeage comme moyen de lutte contre les glossines: recherche du nombre minimal de supports a l’hectare. In: OAU/STRC (see 13: no. 6053), 408 – 412.
- DAVIES, J.E., 1981. Insecticide drift and reinvasion of spray blocks in aerial spraying experiments against morsitans centralis Machado. Bull. ent. Res. 71, 499-508.
- DUNCAN, J., 1963. Post-treatment effects of sublethal doses of dieldrin on the mosquitoAedes aegypti Ann.appl.Biol. 52, 1-6.
- De Haan, C et al., 1997. Finding a balance. In Livestock and the Environment, 115, European Commission Directorate- general for development: Development policy, sustainable development and Natural resources, Brussels.
- Ekwanzala al.,1996. In the heart of darkness: sleeping sickness in Zaire, Lancet, 348, 1427-1430.
- Grant, I. 2001. Insecticides for tsetse and Trypanosomiasis control: is the environmental risk acceptable? Trends in Parasitology 17, (1), 10-14.
- IRVING, N.S. (1968). The absorption and storage of insecticide by the utero larva of the tsetse fly Glossina pallidipes Bull.ent.Res. 58, 221-226.
- JOHNSTONE, D.R., Huntingdon, K.A., Coutts, H.H.,1974. Penetration of spray droplets applied by helicopter into a riverine forest habitat of tsetse flies in West Africa. Agric. Aviat. 16 (3), 71-82.
- JORDAN, A .M. and Trewern, M.A. ,1974. Recent developments in the ecology and methods of control of tsetse flies Glossina spp (Diptera, Glossinidae) a review. Bull. ent. Res. 63, 361-399.
- Kangwagye, T.N., Oliaka, J.E. , Buguma, G. ,1988. Trapping of and ground spraying against Glossina fuscipes in the control of human trypanosomiasis epidemics in N.W. ad S.E. Uganda. In: OAU/STRC (13,(6053), 413 – 421.
- Kupper, W. 1988. The efficiency of various odours in attracting G. tachinoides Results of 2 years’ experiments in northern Cote d’Ivoire. (Abstract only). In: OAU/STRC 13( 53), 407.
- KWAN, W.H. , Gatehouse, A.G., 1978. The effects of low doses of three insecticides on activity, feeding, reproductive performance and survival in Glossinamorsitansmorsitans (Glossinidae). Ent. exp& appl. 23, 201-221.
- KWAN, W.H., Gatehouse, A.G., Kerridge, E. ,1982. Effects of endosulphan on pregnant females of Glossinamorsitansmorsitans Westwood (Diptera: Glossinidae) and their offspring. Bull. ent. Res. 72, 391-401.
- LEE, C. W. Parker, J.D. Kultzre, H, Baldry, D.A.T. Beettany, B.W. ,Tunstal, J. The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachnoides in West Africa. VII. Studies on the physical properties of sprays of endosulphan and deltamethrin applied to G. tachnoides habitats in the R. Komoe Valley, Upper Volta. Tropical Pest Management 26, 377-384.
- LEROUX, J.G. , Platt, D. C. ,1968. Application of dieldrin invert emulsion by helicopter for tsetse control. Pp. 219-229. In Int.Sci.Committee Trypanosomiasis.Res. Twelfth Meeting.Bangui.
- MACLENNAN, K.J.R. , Cook, M.G., 1972. The resting behaviour of Glossina morsitansNewst, in the northern Guinea vegetation Zone in relation to control using insecticides. Entomologist 105: 144-152.
- MAWUENA, K. ,1988. L’utilisation des piegesetecransimpregnesd’insecticide pour la luttecontre la trypanosomiaseanimale: resultatspreliminaires. In: OAU/STRC (13( 6053), 467-471.
- MELLANBY, K. (1937). Experimental work on reproduction in the tsetse fly, Glossina palpalis. Parasitology 29: 131-141.
- MOISIER, B. ,1912. Notes on the haunts and habitats of Glossina tachnoides nearGeidam, Bornu province, Northern Nigeria. Bull.ent.Res 3,195-202.
- Molyneux, D.H, Baldry, D.A.T., Van Wettere, P., Takken, W. and deRaadt, P. The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachnoides in West Africa. 1. Objectives, experimental area and insecticides evaluated. Pest.Artic & News Summ. 24, 391-403.
- Moore, A. al., 1999. Resurgence of sleeping sickness in TamburaCounty, Sudan, Am. J. Trop. Med. Hyg, 61, 315-318.
- NASH, T.A.M. ,1969. Africa’s bane the tsetse fly. Collins and sons Co.LTD OPIYO, E.A., Dolan R.B., Njogu R. Sayer, P.D. ,Mgutu, S.P. ,1988. Tsetse control on Galan ranch. In: OAU/STRC (13: no. 6053) 434-437.
- RIORDAN, E.K. (1987). Insecticide tolerance of pregnant females of Glossina palpalispalpalis(RD) (Diptera: Glossinidae).
- ROGERS, A., Kenyanjui, E.N.F. ,1971. The maintenance of a colony of Glossina pallidipesAnnls of tropical Medicine and parasitology. 66, 267-280.
- SAUNDERS, D.S.,1962. Age determination for female tsetse flies and the age composition of samples of Glossina pallidipes, G. palpalisfuscipesNewst .and G. brevipalpisNewst. Bull. ent. Res. 53, 579-595.
- SAUNDERS, D.S, and Dodd C.W.H.,1972 . Mating, insemination and ovulation in the tsetse fly, Glossinamorsitansmorsitans. J. Insect Physiol. 18, 187-198.
- Shereni, W. 1997. Integrated use of bait techniques for tsetse control in In Proceedings of the 23rd Meeting of the international Scientific Council for Trypanosomiasis Research and Control, 274-287.OAU/STRC.Nairobi, Kenya.
- VALE, G.A. ,1974. The responses of tsetse flies (Diptera: Glossinidae) to mobile and stationary baits. Bull. ent. Res. 64, 488-545.
- Vale, G.A., Hall, D.R. and Gough, A.J.E.,1988. The Olfactory responses of tsetse flies, Glossina (Diptera: Glossinidae), to phenols and urine in the field. Bull. ent. Res. 78 (2), 293-300.
- VAN WETTERE, P., Baldry, D.A.T., Molyneux, D.H., Clarke, J.H., Lee, C.W. and Parker, J.D.,1978. The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachnoides in West Africa. IV. Evaluation of insecticides applied as aerosols. Pest.Artic and News Summ. 24, 435-446. Van Hove, 1997. Sleeping sickness in Zaire, Lancet, 438.
- World Health Organization (WHO) 2001.Committee on African Trypanosomiasis, Scientific working group on African Trypanosomiasis (sleeping sickness), WHO/Tropical Disease Research Unit.

This work is licensed under a Creative Commons Attribution 4.0 International License.





