How to Cite | Publication History | PlumX Article Matrix
Yandri*, Apriyanti, Tati Suhartati and S. Hadi*
Department of Chemistry, Faculty of Mathematics and Natural Sciences University of Lampung, Bandar Lampung - 351 45 Indonesia.
Corresponding Author E-mail:yandrias@unila.ac.id
ABSTRACT: The attempt to increase thermal stability of a-amylase from local bacteria isolate Bacillus subtilis ITBCCB148 was performed by chemical modification using dimethyladipimidate (DMA). The results showed that the native and modified enzymes with DMA (0.3%; 0.5%; and 0.7%) have a similar optimum temperature of 60°C. The thermal stability test done to the native and modified enzymes at 60°C and 60 minutes showed that the native enzyme have a residual activity of 7.7% and t1/2 of 15.4 min, while t1/2 of the modified enzymes were 23.9, 43.3 and 53.3 min. with residual activity of 15.9%, 35.5%, and 42.5, respectively. The chemical modification with DMA to a-amylase has shown to increase the thermal stability of the enzyme between 1.5 – 3.5 times compared to that of the native enzyme.
KEYWORDS: a-amylase; chemical modification; dimethyladipimidate; B. subtilis ITBCCB148
Download this article as:| Copy the following to cite this article: Yandri Y, Apriyanti A, Suhartati T, Hadi S. The Increase of Thermal Stability of A-Amylase from Locale Bacteria Isolate Bacillus Subtilis Itbccb148 by Chemical Modification with Dimethyladipimidate. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Yandri Y, Apriyanti A, Suhartati T, Hadi S. The Increase of Thermal Stability of A-Amylase from Locale Bacteria Isolate Bacillus Subtilis Itbccb148 by Chemical Modification with Dimethyladipimidate. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9136 |
Introduction
α-Amylase is widely applied in many industrial processes both in food and non-food industries. In food industries, for example, this enzyme is used in the production of sugar syrup and fruit juices, while in non-food industries, it is applied in textile industries, especially in the desizing process, a process to remove amylum as textile cover and it is also used in the production of alcohol1.
In order to be applied in industrial sectors, an enzyme requires some specific requirements, including having high thermal stability and wide working pH range1. However, these requirement conditions are not normally owned by any native enzymes, including α-amylase, as native enzyme generally works at physiological conditions and it cannot stand under extreme condition2. To obtain an enzyme that is highly stable, it can be achieved either by isolating thermophilic bacteria directly which is naturally stable or by increasing enzyme stability of a natural enzyme which is not originally stable i.e. a native enzyme which is isolated from any mesophilic microorganisms3,4. According to Mozhaev and Martinek5 to increase the stability of native enzymes three methods are usually applied, which include the immobilization, chemical modification and directed mutagenesis. Chemical modification is the choice method to increase enzyme stability, since either immobilization or directed mutagenesis has some weaknesses.
The modification with bifunctional reagent is a method that is widely applied to increase the native enzyme stability towards pH and extreme temperature6. One of the compounds used as bifunctional reagent is dimethyladipimidate (DMA)7. The DMA has two reactive groups which can cross-link with an enzyme where the bond formed will stabilize the tertiary structure and protect the active site of the native enzymes.
Erarslan and Ertan8 have successfully performed thermobilisation on penicillin G acylase which was obtained from mutant of E. coli using dimethyladipimidate (DMA), dimethylsuberimidate (DMS) and dimethyl-3,3’-dithiopropionimidate (DTBP). The highest increased thermobilisation was observed during the formation of cross-linking with DMA above 50oC. Yandri et al.9 have successfully performed chemical modification with DMA into protease obtained from B. subtilis ITBCCB148, the result obtained showed that the thermal stability of the native enzyme has residual activity of only 1.47%. The enzymes that were modified with DMA with modification degree of 69, 75 and 76% have residual activity of 10.71; 15.87; and 36.36%, respectively, which were much higher than the native enzyme in the order of about 7; 11 and 24 times. Based on these facts, we attempted to elevate the stability of native α-amylase from local bacteria isolate B. subtilis ITBCCB148.
Materials nd Methods
Materials
All chemicals used were of high grade (pro analysis) materials. Local bacteria isolate B. subtilisITBCCB148 was obtained from Microbiology and Fermentation Technology Laboratory, Chemical Engineering Department, Bandung Institute of Technology, Bandung, Indonesia.
Research procedure
The following research phases were done: the production, isolation, purification and characterization of the native enzyme were based on our previous report10.
Activity test of a-amylase and determination of protein content
Activity of a-amylase was determined based on the Iodin method11 and using dinitrosalicylic acid reagent12. The protein content was determined based on the method by Lowry et al.13.
Chemical modification of the native enzyme using dimethyladipimidate (DMA)6
To 10 mL of native α-amylase (containing 0.0305 mg/mL) in 0.1 M buffer phosphate pH 7 was added with solid DMA, so the final concentrations were 0.3%, 0.5% and 0.7% (w/v). The mixture was magnetically stirred at room temperature for 1h.
Characterization of enzyme before and after modification
The characterization of enzyme before and after modification included: determination of optimum pH, kinetic data, and determination of thermal stability.
Determination of optimum temperature of enzyme before and after modification
The determination of optimum temperature of enzyme before and after modification was done by varying the temperature at 55, 60, 65, 70, 75 and 80°C.
Determination of KM dan Vmax values of enzyme before and after modification
The Michaelis-Menten (KM) constant and maximum reaction rate (Vmax) values of enzyme before and after modification were determined by varying the substrate concentration of amylum solution at 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.25%.
The stability test of enzyme before and after modification
The stability of enzyme before and after modification was done based on the known procedure14 which entailed measuring the residual activity of the enzyme after being incubated for 0, 10, 20, 30, 40, 50, and 60 minutes at optimum temperature, where the initial activity of enzyme without heating was given a value of 100%.
Determination of half-life (t½), ki and ΔGi
Determination of ki value (thermal inactivation rate constant) of the native enzyme and the modified enzyme was done using the first order of inactivation kinetics equation (Eq. 1)15:
ln(Ei/E0) = -kit (1)
Where Ei and E0 are the activity of the inactivated and initial forms of the enzyme, respectively: ki is the inactivation rate constant of the enzyme and t is the time.
The denaturation energy change (ΔGi) of the native and modified enzymes was done using Eq. 215:
ΔGi = -RT ln(kih/kBT) (2)
Where ki is the inactivation rate constant of enzyme, kB is the Boltzmann constant, h is Planck’s constant and T is the absolute temperature and R is the universal gas constant.
Results and Discussions
Determination of optimum temperature of the enzyme before and after modification
% activity of α-amylase before and after modification enzymes is presented in Fig. 1. This figure shows that the native enzyme has an optimum temperature of 60°C, while in the modified enzyme, the optimum temperature has shifted from 60°C to 65°C.
The optimum temperature shift in modified enzyme might be due to the presence of DMA molecules inserting into the active site of the enzyme. This condition caused the three dimensional structure of the enzyme to change and the activation energy also changed, as a result it caused the optimum temperature to shift higher. A similar result was also observed by Kazan et al.15.
Fig. 1 also showed that the modified α-amylase with DMA (0.3%, 0.5% and 0.7%) had a higher activity compared to that of the native enzyme at 70-80°C. The activity of the native enzyme significantly decreased at 70°C. The % activity of the modified enzymes with DMA (0.3%, 0.5% and 0.7%) was 71%, 76% and 77%, respectively, while the % activity of the native enzyme was only 23.4%. At higher temperature (80°C), the modified enzymes have % activity of 33%, 44% and 52%, respectively, while the native enzyme was only 2.8%. These results indicated that the modified enzymes with DMA have shown a much higher activity than the native enzyme at higher temperature. It is predicted that the modification process caused the enzyme rigidity to increase and as a result the modified enzyme can stand higher temperature. It was also observed that the highest stability was achieved at the highest concentration of DMA which meant that the higher the concentration of DMA, the higher the stability of enzyme against temperature.
Table 1: The values of ki, t½, and ∆Gi of the native enzyme and after being modified with DMA 0.3%, 0.5%, and 0.7%.
| Enzyme | ki (min.) | t½ (min.) | ∆Gi (kJ mol-1) |
| Native | 0.045 | 15.4 | 101.79 |
| DMA 0.3% | 0.029 | 23.9 | 103.01 |
| DMA 0.5% | 0.016 | 43.3 | 104.66 |
| DMA 0.7% | 0.013 | 53.3 | 105.19 |
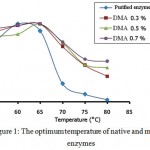 |
Figure 1: The optimum temperature of native and modified enzymes.
|
Determination of KM and Vmax values of enzymes before and after modification
The determination graph of KM and Vmax values of enzymes before and after modification is shown in Fig. 2. Based on Lineweaver-Burk equation, the Vmax (in µmol mL-1 min.-1 ) value of the native enzyme was 90.91 with KM (in mg mL-1 substrate) value of 2.4, while the Vmax and KM values of the modified enzymes with DMA 0.3% = 83.33 and 2.8; DMA 0.5% = 71.43 and 2.7; DMA 0.7% = 62.50 and KM = 2.8.
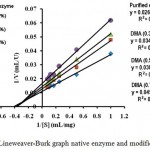 |
Figure 2: Lineweaver-Burk graph native enzyme and modified enzyme.
|
The kinetic data of the native and modified enzymes indicated that there was no big change of their KM values. These results indicated that the enzyme structure has not changed much, especially at the active site of the enzyme, so the enzyme affinity toward substrate has not changed. The Vmax values of all modified enzymes have decreased compared to the native enzyme. This is because the chemical modification with DMA on the enzyme molecules might cause the enzyme to be less flexible in aqueous solution. Unmodified enzyme can interact easily with the substrate, while the presence of DMA molecules changed the availability to interact with substrate which caused the decrease of Vmax. The same observations were also reported by others15,16.
The thermal Stability of enzyme before and after modification
The residual activity of enzyme before and after modification towards temperature was done by incubating each enzyme at 60°C for 60 minutes. Figure 3 shows % residual activity of all enzymes.
Fig. 3 shows that all modified enzymes with DMA have a higher % residual activity compared to that of the native enzyme. The native enzyme has % residual activity of only 7.7% while the modified enzymes with DMA 0.3%, 0.5% and 0.7% have % residual activity of 15.9%; 35.5% and 42.5%, respectively. These results indicated that all enzymes which were modified with different concentrations of DMA have a higher thermal stability than the native one, where in this case, the higher the modification degree, the more stable the enzyme stability. The thermal stability increase was due to the cross-linkage of the enzyme with DMA6.
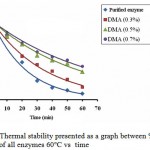 |
Figure 2: Lineweaver-Burk graph native enzyme and modified enzyme.
|
The chemical modification of the native α-amylase obtained from B. subtilis ITBCCB148 was found to have a good effect on the structure stability of the enzyme. The mechanism of how DMA stabilizes the enzyme can be explained as following: (i) the stabilization occurred due to the cross-linking of inter- and intramolecular of DMA to form small circle of enzyme, so it will protect the unfolding in the enzyme tertiary structure; (ii) the cross-link towards the lysine residues in the enzyme surface will reduce the contact of enzyme hydrophobic site with solvent; (iii) the additional formation of hydrogen bonding among the nitrogen of amine groups in DMA molecules with hydrogen atom of carboxyl groups in the enzyme. DMA is a bifunctional reagent that has two functional groups which can be used to bind to two enzyme molecules, as a result there will be more hydrogen bonding formed. According to Kazan et al.6, the higher the modification degree, the more hydrogen bonding formed, so the enzyme rigidity will also increase. This agrees with our result that the higher the concentration of modified agent, the higher the thermal stability of the enzyme.
The thermal inactivation rate constant (ki), half-life (t½) and energy change due to the denaturation value (∆Gi) of the enzyme of enzyme before and after modification
The values of thermal inactivation rate constant (ki), half-life (t½) and energy change due to the denaturation (∆Gi) of the enzyme before and after modification with DMA are presented in Table 1.
The half-lives of all modified enzymes with DMA have increased between 1.5 – 3.5 times compared to that the native enzyme. Stahl17 stated that the half-life of an enzyme will determine the stability of the enzyme. Our result thus agrees with his statement and it was also found that the higher the concentration of DMA, the higher the half-life of the modified enzyme.
The decrease of ki values of the modified enzymes indicated that the decrease of energy change due to the denaturation (∆Gi) has occurred. The stability of the modified enzymes based on the ki values increased 1.5 – 3.5 times compared to the native enzyme. The lower ki value indicated that the enzyme is less flexible in water due to the formation of bonding between DMA with NH2 groups on the side chain of lysine residue on the surface of the enzyme. This will result in decreasing the protein unfolding, so the enzyme structure will be more rigid. The same result was also reported by Kazan et al.6, where the inactivation rate constant of DMA which cross-linked with penicillin G acylase was always lower than the native penicillin G acylase. This was also observed by Yandri et al.9 that the inactivation rate constant of DMA which cross-linked with protease was always lower than the native protease.
The energy change due to the denaturation (∆Gi) of the modified enzymes with DMA was higher than that of the native enzyme. The higher the concentration of DMA, the higher the ∆Gi value obtained. This was because the modified enzyme at higher DMA concentration was more rigid and less flexible in water, so the energy required to denaturate the enzyme was also higher. According to Kazan et al.6, the more penicillin G acylase formed cross-links with DMA, the higher the ∆Gi value. Based on the data in Table 1, it was clear that the ∆Gi values of all modified enzymes with DMA have increased, in which the higher the concentration of DMA used, the higher the increase of the ∆Gi value although it is not significantly high compared to the increase in the thermal stability. A similar result was also found by Yandri et al.9.
In conclusion, the chemical modification using DMA on α-amylase obtained from B. subtilis has successfully increased the thermal stability of the native enzyme. The thermal stabilities of the modified enzymes were increased 1.5 – 3.5 times compared to the native enzyme. The decrease of ki value, the increase of half-life and ∆Gi values showed that the modified enzymes were more stable than the native enzyme.
Acknowledgments
The authors would like to thank The Directorate of Research and Community Services, Directorate General of Higher Education, The Ministry of National Education of Republic of Indonesia that provided funds for this project to be undertaken through the Competency Grant Research Scheme (Penelitian Hibah Kompetensi) 2010 with contract number 394/SP2H/PP/DP2M/VI/2010, 11 June 2010
References
- Vieille C. and Zeikus J. G., Tibtech., 14, 183 (1996).
- Goddettee D.W., Terri C., Beth F.L., Maria L., Jonathan R.M., Christian P., Robert B.R., Shiow S.Y. and Wilson C.R., J. Biotechnol., 28, 41 (1993).
- Wagen E.S., Strategies for increasing the stability of enzymes, in Enzyme Engineering 7, The New York Academy of Sciences, New York, 1-19 (1984).
- Janecek S., Process Biochem., 28, 435 (1993).
- Mozhaev V.V. and Martinek K., Enzyme Microb. Technol., 6, 50 (1984).
- Kazan D., Ertan H. and Erarslan A. Process Biochem., 31, 135 (1996)
- Price N. C., Protein. BIOS Scientific Publishers Limited. Oxford, UK (1996).
- Erarslan A. and Ertan H., Enzyme Microb. Technol., 17, 629 (1995).
- Yandri, Suhartati T., Herasari D. and Hadi S., Nature and Science, 7, 68 (2009).
- Yandri, Suhartati T., Hadi S., Eur. J. Sci. Res, 39, 64 (2010).
- Fuwa H., J. Biochem. (Tokyo), 41, 583 (1954).
- Mandels M., Raymond A. and Charles R., Biotech. Bioeng. Symp., 6, 21 (1976).
- Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J., J. Biol. Chem., 193 (1951).
- Yang Z., Michael D., Robert A., Fang X.Y. and Alan J.R., Enzyme Microb. Technol., 18, 82 (1996).
- Kazan D., Ertan H. and Erarslan A., Appl. Microbiol. Biotechnol., 48, 191 (1997).
- Francis G.E., Delgado C. and Fisher D., PEG-modified proteins in Stability of Protein Pharmaceuticals Part B (Ahern T J & Manning M C, Eds), Plenum Press, New York, 246-247 (1992).
- Stahl S., In: Thermostability of Enzymes (Gupta M.N., ed), Springer Verlag, New Delhi, 59-60 (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.





