Manuscript accepted on : March 12, 2011
Published online on: 28-12-2009
Antioxidant Activity and Fatty Acid Compositions of Arum Dioscoridis Extracts
Huseyin Uguzlar, Esra Maltas and Salih Yildiz
Department of Chemistry, Faculty of Science, Selcuk University, Konya - 42031, Turkey.
Corresponding Author E-mail: esramaltas@hotmail.com
ABSTRACT: In this study, we investigated the antioxidant activity of the extracts of Arum dioscoridis grown in Turkey. The antioxidant activity of the methanolic and acetone extracts from Arum dioscoridis seed was measured by various assays including ferric reducing antioxidant power, cupric reducing antioxidant capacity, scavenging activity of hydrogen peroxide and metal chelating capacity. The methanolic extract showed higher antioxidant activity with 85.4±2.1%. Fatty acid compositions of the methanolic and acetone extracts of Arum dioscoridis were analysed. Data suggested that Arum dioscoridis grown in Turkey may be importance source as natural antioxidant.
KEYWORDS: Arum dioscoridis; Antioxidant activity; FRAP; CUPRAC; fatty acid
Download this article as:| Copy the following to cite this article: Uguzlar. H, Maltas. E, Yildiz. S. Antioxidant Activity and Fatty Acid Compositions of Arum Dioscoridis Extracts. Biosci Biotechnol Res Asia 2011;8(1) |
| Copy the following to cite this URL: Uguzlar. H, Maltas. E, Yildiz. S. Antioxidant Activity and Fatty Acid Compositions of Arum Dioscoridis Extracts. Biosci Biotechnol Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9216 |
Introduction
Arum dioscoridis growing in Asia and Europen is one of the species of Araceae family. For the centuries, it has been used as a traditional plant for disease treatment (1). In literature, only a few study has been reported about Arum dioscoridis. S. Janakat and O. Al-Thnaibat (2) studied on antilipoperoxidative activity of the methanolic extract of Arum dioscoridis leaves against lipid peroxidation (LPO) induced by a rat liver homogenate model. They examined LPO levels of their leaves on the rats. In addition, Ido Izhaki (3) determined aminoacid and protein contents of Arum dioscoridis grown in East Mediterranean.
The protective effect of the plant such as Arum dioscoridis is directly outcome from their chemical component such as phenolic acids, flavonoids, polyphenolic compounds, linoleic acid and linolenic acid. The plant extract can be used in food processing, pharmaceutical, nutriceutical and food additives because of high antioxidant activity. The antioxidant compounds such as phenolic acids, flavonoids, carotenoids and anthocyanidin in the plant extract scavenge free radicals such as superoxide (O2–), hydrogene peroxide (H2O2), hyrodxyl (OH–), nitrogen radical (NO–), peroxynitrite (ONOO–) and hypochloride (OCl–) coming from metabolic pathways of human. There are many reports associated with many diseases including artherosclerosis, diabetes, immun deficiency diseases and aging on biological effect of the plant extract (4-8). However, ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) may be helpful in treating a variety of health conditions such as heart disease, diabetes mellitus, arthritis, osteoporosis, asthma, colon cancer, breast cancer and prostate cancer associated with oxidative damage of the free radicals. Fatty acids with conjugated double bonds markedly increases rate of the oxidation related to polyunsaturated fatty acids with methylene-interrupted double bonds (9-14).
Any study hasn’t reported on antioxidant activity of Arum dioscoridis from Turkey in the literature. It’s known that climatical changes cause changes on morphological and chemical structure of the herbals (15). In this study, we evaluated the antioxidant activity of the extracts of Arum dioscoridis grown in the South of Turkey by the methods including cupric reducing antioxidant capacity (CUPRAC), ferric reducing antioxidant power (FRAP), metal chelating capacity and scavenging activity of hydrogen peroxide. In addition to the antioxidant analysis, phenolic contents of the extracts were also determined by Folin Ciocalteu method. The fatty acid compositions of Arum dioscoridis extracts were analysed by using gas chromatography.
Materials and Method
Plant material and extraction
Arum dioscoridis was grown in Antalya in the South of Turkey. Seed of the plant was collected in June 2008 and dried. The plant collected identified at Akdeniz University, Departmant of Biology, Antalya, Turkey. The plant materials were cleaned, washed, dried and carefully powdered. All samples were kept in tightened light-protected containers. Dried leaves (50 g) were powdered and extracted with methanol and acetone for 6 h at 30°C using an orbital shaker (16). Extract solutions were filtered through a filter paper (Whatman No.1) and the solvent was evaporated under vacuum to 10 mL then dried at –50°C in a lyophiliser. Yield of the extracts of methanol and acetone were 16 and 9 (w/w %), respetively.
Ferric-reducing antioxidant power
Ferric-reducing antioxidant power of Arum dioscoridis extracts was determined according to the method of Oyaizu (17). 0.5 mL of of the extract at various concentrations (0.25, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 mg mL-1) in 1mL of methanol was mixed with 2.5 mL of phosphate buffer (1%, pH 6.6) and 2.5mL of 1% (w/v) potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50°C for 20 minutes, then 2.5mL of trichloroacetic acid (10%, w/v) was added to the mixture, which was then centrifugation at 650g for 10 minutes. Finally, 2.5 mL of the supernatant solution was transferred to another test tube with 5 mL of distilled water and 1 mL of 0.1% (w/v) FeCl3. Absorbance was recorded at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power (18). The samples were run in triplicate.
Cupric reducing antioxidant capacity
The cupric reducing antioxidant capacity of Arum dioscoridis extracts was determined according to the method of Apak et al., (19). To a test tube was added 1 mL of mixture of 10 mM Cu (II), 7.5 mM neocuprine, and NH4Ac buffer (1 M, pH 7.0) solutions were added to 0.5 mL extract (0.01-0.5 mg mL-1) followed by water was then added (total volume, 4.1 mL) and mixed well. The mixture was incubated for 1 h, then the absorbance at 450 nm was recorded. CUPRAC procedure was applied to each Trolox solution at various concentrations and absorbance was recorded at 450 nm. Calibration curve (0.05-0.5 mg mL-1) was drawn for Trolox. CUPRAC values of the extracts equvailent to mg Trolox per mg dry extract (CUPRACTEAC) were measured from standard curve equation (y = 4.923x + 0.075, mg mL-1 TEAC, R2 = 0.998) of Trolox calibration curve (21). The samples were run in triplicate.
Metal chelating capacity
The metal chelating capacity of Arum dioscoridis extracts was determined by the method described by Que et al. (22). Extract solutions (0.1-1 mg mL-1) were prepared from the dry extract. 1 mL of the extract was mixed with 1 mL of methanol, 0.1 mL of 2 mM FeCl2×4H2O and 0.2 mL of 5 mM ferrozine. After 10 minutes, absorbance was recorded at 562 nm by Shimadzu 1700 UV–Vis Spectrophotometer (Kyoto, Japan). The ability of the extracts to chelate ferrous ions (I%) was calculated as follows (Gülçin, Oktay, Kireçci & Kührevioğlu, 2003):
I% = [(Ablank – Asample) /Ablank]×100
where Ablank is absorbance of the blank, Asample is absorbance of the extract or standard. Rutin was used as comparative standard. The samples were run in triplicate.
Scavenging ability of hydrogen peroxide
The ability of Arum dioscoridis extracts to scavenge hydrogen peroxide (H2O2) was described by the method of Benkeblia (23). In this assay, absorbance of 2 mM hydrogen peroxide solution in phosphate-buffered saline (pH 7.4) was recorded at 230 nm by UV-Visible spectrophotometer. 0.6 mL of the extracts (0.01-0.5 mg mL-1) was added to 0.5 mL of H2O2 solution and the hydrogen peroxide concentration was determined spectrophotometrically at 230 nm. An aliqout of extract was added to H2O2 solution (0.6 mL) and absorbance of the hydrogen peroxide at 230 nm was read after 10 minutes against a blank solution containing extract (1 mL) in PBS without H2O2. The scavenging ability of hydrogen peroxide was determined by using following equation by Gülçin et al. (24)
I % = [(Ablank – Asample) /Ablank]×100
where Ablank is absorbance of the blank (extract in PBS without H2O2), Asample is absorbance of the extract or standard in the presence of H2O2. Quercetin and rutin were used as comparative standards. The samples were run in triplicate.
Fatty acid composition
The fatty acid compositions of Arum dioscoridis extracts were analysed chromatographically by converting to methyl esters. The methanolic and acetone extracts of Arum dioscoridis were derived with 5 % sodium methoxide overnight. Fatty acid methyl esters of both extracts were analysed on a Shimadzu GC 5050 gas chromatography (Shimadzu, Kyoto, Japan). The chromatographic column for the analysis was a Cp Wax 52 CB capillary column (50 m\0.32 mm, 1.2 µm). Helium at a flow rate of 10 psi min-1 was used as carrier gas and the temperature was kept at 240°C for 15 minutes. All samples were analysed in three replications.
Statistical analysis
The statistical analysis was carried out by using OriginPro 7.5 software. One way ANOVA was applied to data and results were compared by using Tukey test. A difference was considered to be statistically significant when the p-value is equal to 0.05 (p = 0.05).
Result and Discussion
Ferric-reducing antioxidant power of the methanolic and acetone extracts from Arum dioscoridis was measured spectrophotometrically at 700 nm. The antioxidant action of the ferric reducing agent is based on electron-donating ability of the antioxidants by the potassium ferricyanide reduction. The antioxidant compounds such as phenolic acids, flavonoids and polyphenolic compounds in the extracts reduce the ferric ion/ferricyanide complex to the ferrous form, the Perl’s Prussian blue complex (25). Figure 1 showed the plot of the reducing power of the methanolic and acetone extracts and BHA, BHT and α-tocopherol as standards related to the extract concentration in the FRAP method. Approximately a linear increase in absorbance was observed over the concentration ranged 0.05–0.5 mg mL-1 of the methanolic extract. The methanolic extract had significantly stronger (p = 0.05) ferric reducing antioxidant power than that of the acetone extract at an extract concentration of 0.5 mg mL-1. However, the reducing power of BHA and BHT and α-tocopherol was higher than that of the methanolic extract of Arum dioscoridis. High reducing power of the methanolic extract could be due to the presence of high amount of the phenolic compounds, compared to that of the acetone extract.
The antioxidant activity of the plant extract is attributed to the phenolic and flavonoid compounds that varies on the plant tissue such as seed, leaves and root. However, plant species, geographical factors and climatical changes effect the distrubition of the phenolics and flavonoids, result in different antioxidant activity (15,25). Antioxidant activity of the antioxidants or the plant extracts can be followed spectroscopically through inhibition of free radical formation by chelating transition metal ions (4). The phenolics and flavonoids in the extracts donate hydrogen atom by acting as chain-breaking antioxidants The chelating ability of the methanolic and acetone extracts from Arum dioscoridis on ferrous ion was determined spectroscopically at 562 nm. Chelating ability of the standard (92.1±1.1%), rutin, was approximately similar with that of the methanolic extract (85.4±2.1%) whereas chelating ability of the methanolic extract was more effective than that of the acetone extract (42.3±1.2%) at an extract concentration of 0.5 mg mL-1 (Figure 2). High chelating ability of the methanolic extract from Arum dioscoridis is attributed high phenolic and flavonoid content.
Table 1: Fatty acid content of Arum dioscoridis extracts as a percent of total (%).
| Fatty acids
(%)1 |
Methanolic extract (%)1
|
Acetone extract
|
Retention time (min.) |
| dimethyl ester | – | 4.72±0.19 | 12.4 |
| Levulinic acid ME | 19.58±1.05 | 6.65±0.39 | 15.2 |
| Butanedioic acid | 2.02±1.17 | 1.78±0.21 | 15.4 |
| methyl dimethyl ester | |||
| Hepta 2,4-dienoic | 16.16±2.12 | 2.00±0.55 | 23.4 |
| acid ME | |||
| Hexadecanoic | |||
| acid 2-hydroxy ME | – | 5.50±0.75 | 59.7 |
| Σ Acids | 37.76 | 20.65 | |
| C 12:0 | – | 0.46±0.06 | 23.6 |
| C 14:0 | – | 0.9±0.04 | 28.4 |
| C 16:0 | 16.11±1.26 | 23.60±2.34 | 43.8 |
| C 18:0 | 1.70±0.27 | 3.12±0.78 | 54.7 |
| Σ SFAs2 | 17.81 | 28.08 | |
| C 16:1 | 3.31±1.23 | 4.15±1.05 | 45.4 |
| C 18:1 | 17.38±1.79 | 21.69±1.39 | 56.0 |
| Σ MUFAs2 | 20.69 | 25.84 | |
| C 18:2 | 18.03±2.17 | 21.90±2.65 | 58.1 |
| C 18:3 | 3.12±0.32 | 4.30±0.21 | 60.9 |
| Σ PUFAs2 | 21.15 | 26.2 |
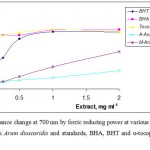 |
Figure 1: Absorbance change at 700 nm by ferric reducing power at various concentrations of the extracts from Arum dioscoridis and standards, BHA, BHT and α-tocopherol on ferrous ions. |
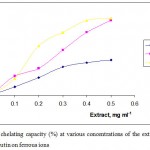 |
Figure 2: Metal chelating capacity (%) at various concentrations of the extracts from Arum dioscoridis and rutin on ferrous ions.
|
The scavenging ability of Arum dioscoridis extracts on hydrogen peroxide was determined at 230 nm. The absorbance change of hydrogen peroxide in the presence of the extracts was recorded by UV-spectrometer and compared with absorbance change of that in the presence of rutin and quercetin as the standards. As shown in Figure 3, the scavenging ability of the extracts and the standards was in the order of quercetin (84.09±1.52%) > rutin (74.53±1.14%) > methanolic extract (68.21±1.76%) > acetone extract (31.27±1.09%) at concentration of 0.5 mg mL-1. The results indicated that the scavenging ability of the methanolic extract found to be higher than that of the acetone extract. The main role of the phenolic compounds as the scavengers of the free radicals is emphasized in several reports (25-26). Antioxidative properties of the essential oils and various extracts from many plants are of great interest in both academic researches and food industry, since their possible use as natural additives emerged from a growing interest to replace synthetic antioxidants by natural ones.
The antioxidant action of the reducing agent is based on terminating the radical chain by donation of a hydrogen atom in the CUPRAC method (25). The Trolox equivalents antioxidant capacity (TEAC) is defined as equvailents to mg mL-1 Trolox by the CUPRAC method using copper(II)-neocuproine reagent. The CUPRACTEAC values of the extracts were found from the absorbance of the solutions allowed to stand for 30 minutes at room temperature after copper(II)-neocuproine reagent addition at 450 nm. CUPRACTEAC values were simply calculated by the standard curve equation obtained from the calibration curve of Trolox. The CUPRAC values equivalent to Trolox were found to be as 1.55±0.23 mg mL-1 in the methanolic extract and as 1.36±0.18 mg mL-1 in the acetone extract. Many researchers have reported that the total phenolic and flavonoid contents of the extracts play important role in the redox potential of the extracts (25-27).
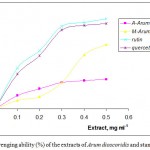 |
Figure 3: Scavenging ability (%) of the extracts of Arum dioscoridis and standards by H2O2.
|
Fatty acid composition of Arum dioscoridis was analysed in the methanolic extracts acetone extract of Arum dioscoridis (Fig. 4 and 5). Table 1 indicated monounsaturated fatty acids (MUFAs), saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs) in the extracts. The results indicated that there was a minor difference on MUFAs, PUFAs and SFAs values of both extracts. The main compounds of the extracts of canola genotypes were linoleic acid, palmitic acid and oleic acid. Fatty acids such as ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) were effective on the several diseases such as heart disease, diabetes, arthritis, osteoporosis, asthma, colon cancer, breast cancer and prostate cancer associated with the oxidative damage of free radicals (28-30).
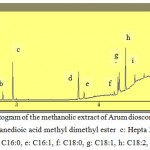 |
Figure 4: Chromatogram of the methanolic extract of Arum dioscoridis , a: Levulinic acid ME b: Butanedioic acid methyl dimethyl ester c: Hepta 2,4-dienoic acid ME d: C16:0, e: C16:1, f: C18:0, g: C18:1, h: C18:2, i: C18:3, |
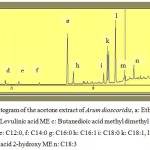 |
Figure 5: Chromatogram of the acetone extract of Arum dioscoridis, a: C14:0, b: C16:0, c: C16:1, d: C18:0, e: C18:1, f: C18:2, g: C18:3.
|
In conclusion, studying with Arum dioscoridis may be of great interest, since its bioactive properties and medicinal effects have been known in centuries. Owing to its excellent protective features related to the antioxidant activity, the extracts of Arum dioscoridis could be concluded as a natural source that can be freely used as a herb in the food industry. Therefore, several analysis such as chromatographic and mass spectrometric analysis should be taken for quality and safety control of the extracts and the protection of these plant species. However, the antioxidant activity of Arum dioscoridis grown in Turkey hasn’t been reported in the literature. So, this study can be considered as the first report on in vitro antioxidant properties of the methanolic and acetone extract of Arum dioscoridis from Turkey.
Acknowledgement
We thank Scientific Research Foundation of Selcuk University (BAP) for providing foundation with 09201034 of project number. This study is a part of Hüseyin Uguzlar’s Msc thesis.
References
- Ali-Shtayeh M. S., Yaniv Z., Mahajna J., J. Ethnopharm., 73, 221-232 (2000).
- Janakat S. and Al-Thnaibat O., J. Food Qual., 31(1), 1-12 (2008).
- Izhaki I. J. Chem. Ecol., 24: 1333-1345 (1998).
- Vaya J. and Aviram M., Current Med. Chem. Imm. Endoc.. & Metabolic Agents, 1, 99-117 (2001).
- Wada L. and Ou B., J. Agric. Food Chem., 50, 3495-3500 (2002).
- Halliwell B. and Gutteridge J. M. C., Oxford University Press: Oxford, U.K (1989).
- Williams C. A., Harborne J. B., Geiger H., Hault J. R. S., Phytochem., 51, 417-423 (1999).
- Stampfer M. J., Hu F. B., Manson J. E., Rimm E. B., Willett W. C., New England Journal Medicine, 343 (1), 16-22 (2000).
- Tsai W. S., Nagawa H., Kaizaki S., Tsuruo T., Muto T., J. Gastroenterol., 33, 206-212 (1998).
- Tsujikawa T., Satoh J., Uda K., Ihara T., Okamoto T., Araki Y., J. Gastroenterol., 35(2), 99-104 (2000).
- Shils M. E., Olson J. A., Shike M., Ross A. C., Modern nutrition in health and disease. 9th ed. Baltimore, Md: Williams & Wilkins pp. 90-92 and pp. 1377-1378 (1999).
- Kruger M. C. and Horrobin D. F. Prog. Lipid Res., 36, 131-151 (1997).
- Madhavi N. and Das U. N., Cancer Lett., 8, 31-41 (1994).
- Simopoulos A. P. Amrc. J. Clin. Nutr., 70 (3), 560-569 (1999).
- Perry N. B., Anderson R. E., Brennan N. J., Douglas M. H., Heaney A. J., Mcgimpsey J. A. and Smallfield B. M., J. Agric. Food Chem., 47, 2048–2054 (1999).
- Gulcin I., J. Food Sci. Nutr., 56, 491-499 (2005).
- Oyaizu M., Jpn. J. Nutr., 44, 307-315 (1986).
- Gulcin I., Elmastas M. and Aboul-Enein H. Y., Phytotherapia Res., 21 (4), 354-361 (2007).
- Apak R., Guclu K., Ozyurek M., Karademir S. E. and Ercag E., Int. J. Food Sci. Nutr., 57(5/6), 292-304 (2006).
- Gulcin I., Tel A. Z. and Kirecci E., Int. J. Food Proper., 11(2), 450-471 (2008).
- Gulcin I., J. Enzyme Inh. Med. Chem., 23(6), 871–876 (2008).
- Que F., Mao L., Zhu C. and Xie G., LWT, 39, 111–117 (2006).
- Benkeblia N., Braz. Arch. Biol. Tech., 48, 753-759 (2005).
- Gülçin I., Oktay M., Kireçci E. and Kührevioğlu Ö.I. Food Chem., 83, 371–382 (2003).
- Chu Y. H., Chang C. L. and Hsu H. F., J. Sci. Food Agric., 80, 561-566 (2000).
- Apak R., Güçlü K., Demirata B., Özyürek M., Çelik S. E., Bektaşoğlu B., Ber K. I. and Özyurt D., Molecules, 12, 1496-1547 (2007).
- Macdonald H. B. J. Am. Coll. Nutr., 19, 111S–118S (2000).
- Rudin M. D. and Felix C., Omega-3 Oils; A practical Guide. US: Avery, (1996).
- Cahoon E. B., Carlson T. J., Ripp K. G., Schweiger B. J., Cook G. A., Hal S. E. and Kinney A. J., PNAS, 22, 12935-12940 (1999).
- Qi B., Fraser T., Mugford S., Dobson G., Sayanova O., Butler J., Napier J. A., Stobart A. K. and Lazarus C. M., Natural Biotech. 22, 739-745 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.





