Manuscript accepted on : May 15, 2011
Published online on: --
Bioremediation of Tannery Effluent by Selected Fungal Species
Deepa1*, K. Valivittan2, Indira Vincent3 and C. S. Tharadevi4
1,2Department Of Biotechnology, St. Peter’s University, Avadi, Chennai - 600 054 India.
3Department Of Zoology, Presidency College, Chennai - 600 005 India.
4Department Of Zoology, Sree Ayyappa College For Women, Nagercoil - 629 003 India.
Corresponding Author E-mail: reghudeep09@gmail.com
ABSTRACT: Microbial population has amazing enzymatic and metabolic potential to degrade a variety of organic compounds. Eighteen species of fungi were isolated from leather tanning effluents from Thirumudivakkam, Chennai. Out of these four dominant species of fungi like Aspergillus niger, Aspergillus terreus, Paecilomyces varioti and Phanerochaete chrysosporium were selected and used for bioremediation of effluents. Individual and mixed cultures of fungi were used to control pH, BOD, COD, TSS and TDS in the tannery effluent. The biodegradation of tannery effluent was estimated at two different periods of incubation, on the 6th and 12th day. Among all experiments, biodegradation by using mixed culture of fungi showed better results than that by individual species.
KEYWORDS: Tannery effluents; Bioremediation; Colony forming units; Fungal species; pH; BOD; COD; TSS; TDS
Download this article as:| Copy the following to cite this article: Deepa, Valivittan K, Vincent I, Tharadevi C. S. Bioremediation of Tannery Effluent by Selected Fungal Species. Biosci Biotech Res Asia 2011;8(1) |
| Copy the following to cite this URL: Deepa, Valivittan K, Vincent I, Tharadevi C. S. Bioremediation of Tannery Effluent by Selected Fungal Species. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9286 |
Introduction
Microbes in the environment play an important role in cycling and destroying pollutants through biodegradation. Bacteria and fungi are the chief agents for biodegradation, while yeast, algae, diatoms, some plants and animals also metabolize chemicals (Alexander, 1981)1. Since the complete biodegradation of organic chemicals in the natural ecosystem is primarily due to microorganisms, biotechnological applications employ microbes or their enzymes for waste treatment (Ninnekar, 1991)2.
Scientists are currently concentrating on the isolation of an organism and its degradation capacity of certain organic and inorganic compounds and metals used in various industrial processes (Radha, 1995)3. More et al., (2001)4 have suggested that bioremediation will be a cleaner way to treat the effluents as they operate under milder conditions with minimum generation of by-products. Only a limited number of attempts have been made towards this in the past.
Based up on the above studies the present study was executed to study the biodegrading capacity of native fungi present in the fungi population of tannery effluent individually and also in mixed culture.
Materials and Methods
The area selected for the present study is situated at Thirumudivakkam, 5 kms away from Pallavaram, Chennai.The effluent samples for the current study were collected from the final discharge point where in effluent from all the stages of processing are released together from a tannery in Thirumudivakkam. The effluent samples were collected in polythene containers of 5 litres capacity and brought to the laboratory with due care and stored at 200 C until further analysis. The period of collection was 12 months from May 2010 to April 2011. pH, BOD, COD, TSS ,TDS were analysed following standard methods (APHA,2005)5.
Isolation, identification and culture of Fungi from tannery effluent
Tannery effluents collected were used for the analysis of mycoflora. The fungi were isolated using dilution plating method. Potato Dextrose Agar (PDA) was used as culture medium (Booth, 1971)6.The petri dishes containing the effluent sample in PDA were incubated at room temperature 28˚C ±2˚C in a glass chamber for the isolation of fungi for a period of 4 to 5 days the growing colonies were counted and identified up to species level. Wherever necessary the colonies were sub-cultured in Czapek’s Dox Agar (CDA) for species identification. Lactophenol and lactophenol with cotton blue (for hyaline molds) were used for preparing slides for examination and the slides were sealed with DPX mountant for future use. The fungi mounted in lactophenol cotton blue were observed through light microscope and were identified using standard manuals (Gilman (1967)7; Subramanian (1971)8; Ellis (1971)9; Barnett and Hunter (1972)10.
The results obtained were presented as (Colony forming units) “cfu/ml” and “percent contribution”

The term “percent contribution” refers to the contribution of individual species to the total, and is calculated as follows,
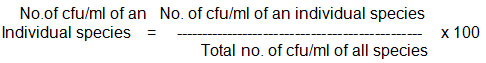
After identification of the fungi, mass culture was carried out in the laboratory to be used for biotreatment of the tannery effluent. Fungi such as Aspergillus niger, Aspergillus terreus, and Paecilomyces varioti were cultured in mass using the Malt Extract Agar medium. Whereas, the fungus, Phanerochaete chrysosporium was grown in the specific medium containing Thiamine hydrochloride (Kirk et al., 1976)11.
Biodegradation of tannery effluent using individual culture: Mycelial mat of fungi in liquid culture was recovered and washed in sterile distilled water. Approximately 10gms (fresh weight) of mycelia of Aspergillus niger, Aspergillus terreus, Paecilomyces varioti and Phanerochaete chrysosporium were transferred to experimental jars separately containing 500ml of raw (100%) tannery effluent. Similarly mycelial mat of mixed fungi (Aspergillus niger, Aspergillus terreus, Paecilomyces varioti and Phanerochaete chrysosporium) grown in mass culture was recovered and washed in sterile distilled water and transferred to experimental jars separately containing 500ml of raw (100%) tannery effluent. All jars were kept in a shaker and maintained at 28˚C± 2˚C. A few required physico-chemical parameters such as pH, BOD, COD, TSS and TDS were estimated in the tannery effluent before and after an interval of 6 days and 12 days to check the degradation process by the microbes in each jar.
Statistical Analysis
The data obtained from the various experiments were subjected to statistical analysis in order to understand the validity of the data.
The percentage change was calculated between the control and experimental for the various experiments using the formula

Results and Discussion
Isolation and identification of Fungi:
Eighteen species of fungi were isolated and identified from the tannery effluent during the month of May 2010 to April 2011. The species included Scopulariopsis brevicaulis, Monilia sitophila, Aspergillus niger, Aspergillus flavus, A. terreus, A. versicolor, A. japonicus A. fumigatus, A. chevalieri, A. nidulans, Trichoderma sp., Curvularia lunata ,Syncephalastrum racemosum, Mucor racemosum, Ascidia corymbifera, Humicola grisea, Phanerochaete chrysosporium and Paecilomyces varioti. From the isolated fungi species four most common forms (Aspergillus niger, A. terreus Phanerochaete chrysosporium and Paecilomyces varioti) were selected and used for the biodegradation experiments.
Biodegradation of tannery effluent using individual species of fungi and mixed culture on 6 th and 12th day
pH of tannery effluent after biodegradation
Biodegradation of tannery effluent in two different days at an interval of 6 days ie., on 6th and 12th day of incubation by individual fungus and mixed culture of fungi with regard to pH is shown in Table 1. On the 6th day of biodegradation the pH increased to 6.88 in mixed culture, followed by Aspergillus terreus (6.77).Corresponding percentage change of +52.55 % and +50.11% respectively.On 12th day of biodegradation, change in pH obtained by individual species did not vary much (7.53-7.75). However pH value obtained (7.77) by mixed culture was a little higher than those for individual species. Between the 2 intervals of incubation for change in pH by four different microbes and mixed culture, it may be pointed out that the pH reached neutral. The increase in pH from acidic to neutral is statistically significant at p<0.001 level.
Table 1: pH of raw tannery effluent on the 1st day and after biodegradation using individual fungi and mixed culture on 6th and 12th day
| Days | Mean ± S.D. &
% Change |
Raw Effluent (Control) | P.
varioti |
A.
niger |
A.
terreus |
P.
chrysosporium |
Mixed Culture |
| 1st day | Mean ± S.D. | 4.51 ± 0.06 | – | – | – | – | – |
| 6th day | Mean ± S.D. | 4.54 ± 0.05 | 6.36 ± 0.04* | 6.21 ± 0.05* | 6.77 ± 0.06* | 6.46 ± 0.05* | 6.88 ± 0.06* |
| %
Change |
+0.67
% |
+41.02% | +37.69% | +50.11% | +43.24
% |
+52.55% | |
| 12th day | Mean ± S.D. | 4.57 ± 0.04 | 7.57 ± 0.05* | 7.53 ± 0.05* | 7.75 ± 0.05* | 7.64 ± 0.05* | 7.77 ± 0.04* |
| %
Change |
+1.33
% |
+67.85% | +66.96% | +71.84 % | +69.40
% |
+72.28% |
P<0.001
Table 2: BOD of raw tannery effluent on the 1st day and after biodegradation using individual fungi and mixed culture on 6th and 12th day
| Days | Mean ±
S.D. & % Change |
Raw Effluent (Control) | P.
varioti |
A. niger | A. terreus | P. chrysosporium | Mixed Cultue |
| 1st | Mean ±
S.D. |
2568±
26.4 |
– | – | – | – | – |
| 6th | Mean ±
S.D. |
2508±
22.4 |
629± 22.09* | 896±
34.6* |
1121± 12.9* | 920±
31.0* |
775±
16.5* |
| % Change | -2.34
% |
-73.05
% |
-65.11
% |
-56.35
% |
-64.17
% |
-69.82
% |
|
| 12th | Mean ±
S.D. |
2430±
22.3 |
322±
8.1* |
585±
11.4* |
762± 8.1* | 485±
8.6* |
255±
8.2* |
| % Change | -5.37
% |
-87.46
% |
-77.22
% |
-70.33
% |
-81.11
% |
-90.07
% |
P<0.001
Table 3: COD of raw tannery effluent on the 1st day and after biodegradation using individual fungi and mixed culture on 6th and 12th day
| Days | Mean ± S.D. & % Change | Raw Effluent (Control) | P.
varioti |
A. niger | A. terreus | P. chrysosporium | Mixed Cultue |
| 1st | Mean ±
S.D. |
7127±
11.4 |
– | – | – | – | – |
| 6th | Mean ±
S.D. |
7111±
12.3 |
2842±
27.8* |
4595±
7.9* |
2996±
28.7* |
3102±
32.2* |
2355±
17.8* |
| % Change | -0.22
% |
-60.12
% |
-35.53
% |
-57.96
% |
-56.48
% |
-66.96
% |
|
| 12th | Mean ±
S.D. |
7095±
11.2 |
1175± 13.5* | 1228± 10.2* | 1449± 28.2* | 1023± 12.4* | 987± 22.7* |
| % Change | -0.45
% |
-83.51
% |
-82.76
% |
-79.67
% |
-85.65
% |
-86.15
% |
P<0.001
Table 4: TSS of raw tannery effluent on the 1st day and after biodegradation using individual fungi and mixed culture on 6th and 12th day
| Days | Mean ± S.D. & % Change | Raw Effluent (Control) | P.
varioti |
A.
niger |
A. terreus | P. chrysosporium | Mixed Cultue |
| 1st | Mean ± S.D. | 4958± 24.9 | – | – | – | – | – |
| 6th | Mean ±
S.D. |
4893±
21.5 |
1128±
11.6* |
2347± 25.6* | 2319±
12.4* |
2482±
15.9* |
1046± 11.4* |
| % Change | -1.31
% |
-77.25
% |
-52.66
% |
-53.23
% |
-49.94
% |
-78.90
% |
|
| 12th | Mean ±
S.D. |
4812±
12.2 |
982± 16.1* | 1294± 13.9* | 1247± 24.7* | 1363±
12.9* |
862± 11.4* |
| % Change | -2.94
% |
-80.19
% |
-73.90
% |
-74.85
% |
-72.51
% |
-82.61
% |
P<0.001
Table 5: TDS of raw tannery effluent on the 1st day and after biodegradation using individual fungi and mixed culture on 6th and 12th day
| Days | Mean ± S.D. & % Change | Raw Effluent (Control) | P.
varioti |
A. niger | A. terreus | P.
chrysosporium |
Mixed Culture |
| 1st | Mean ±
S.D. |
6780±
20.0 |
– | – | – | – | – |
| 6th | Mean ±
S.D. |
6720±
21.0 |
6162±
31.6* |
5955±
29.0* |
6069±
34.0* |
5998±
31.7* |
4025±
26.9* |
| %
Change |
-0.88
% |
-9.11
% |
-12.17
% |
-10.49
% |
-11.53
% |
-40.63
% |
|
| 12th | Mean ±
S.D. |
6642±
29.15 |
5655±
31.9* |
5412±
32.19* |
5535±
25.1* |
5649±
42.2* |
3142±
31.10* |
| %
Change |
-2.04
% |
-16.59% | -20.17
% |
-18.36
% |
-16.68
% |
-53.66
% |
P<0.001
BOD of Tannery Effluent After Biodegradation
Biodegradation of tannery effluent on two different days of interval (6 and 12 days) by the individual fungus and mixed culture for BOD is shown in table 2.On the 6th day, there was drastic change in degradation by P.varioti (-73.05%) followed by the mixed culture (-69.82%), A. niger (-65.11%), P .chrysosporium (-64.11%) and A. terreus (-56.35%) indicating the biodegradation ability of the microbes. While on the 12th day the efficiency of biodegradation increased to 87.46% by P. varioti and -90.07% by the mixed culture. The reduction in BOD in the bio-treated effluent is statistically significant P<0.001.
COD of tannery effluent after biodegradation
Biodegradation of raw tannery effluent on 6th and 12th day by the individual fungus and mixed culture for COD is presented in table 3. On the 6th day, P. varioti recorded a reduction of -60.12% whereas in the mixed culture treatment the reduction was to the tune of -66.96%. On the 12th day P. chrysosporium marked a reduction of -85.65%, and the mixed culture recorded the reduction of -86.15%. Decrease in COD in the biodegraded effluents is statistically significant P<0.001.
TSS of tannery effluent after biodegradation
Biodegradation of raw tannery effluent on the 6 th and 12th day of incubation by the individual fungus and mixed culture for TSS is shown in table 4. On the 6th day mixed culture showed a reduction of -78.90% of TSS followed by P. varioti (-77.25%). On the 12th day the reduction of TSS was -74.85% by A .terreus , -73.90% by A. niger, -72.51% by P. chrysosporium and -80.19% by P. varioti. Mixed culture reduced -82.61% of the TSS. Decrease in TSS in bio-treated effluent is statistically significant P<0.001.
TDS of tannery effluent after biodegradation
The observations noticed in the biodegradation of TDS in the raw tannery effluent in two different days of intervals (6 and 12 days) by the individual fungus and mixed culture is shown in table 5. On the 6th and 12th day the percentage changes shown by A. niger is -12.17 and – 20.17% respectively. In the case of mixed culture the reduction was- 40.63% on the 6th day and -53.66% on the 12th day. Reduction is TDS in bio-treated effluent is statistically significant p<0.001.
Discussion
Tannery effluent is rich in organic and inorganic nutrients, which will support the growth of the microbial population. Bacteria and fungi are the chief agents for the biodegradation of organic compounds. 18 species of fungi are identified in the present investigation. Rao & Rao (2000)12 reported the presence of over 25 species of fungi in the tannery effluents.
In the present study the dominant mycoflora noticed in the tannery effluent are Aspergillus terreus, Phanerochaete chrysosporium, Paecilomyces varioti and Aspergillus niger. Radha (1995)3, pointed out that the presence of native microbes in tannery effluent can be successfully exploited to remove the pollutants, a technique which is more economically and industrially effective. Results showed the effectiveness of this methodology in bringing about favourable reductions in the levels of physico-chemical parameters like pH, BOD, COD, TDS, and TSS. Present study indicates that the pH changed from acidic to near neutral, on biodegradation by all the four candidate species and by mixed culture. Mixed culture with a percentage change of about +72.28%, and A. terreus (+71.84%) showed more efficiency in changing the pH from acidic to alkaline side.It may be notified that P.chrysosporium and A. terreus have the ability to change the pH from acidic to neutral, than the other two fungi indicating their efficiency. It has been recommended that they are the most widely studied organisms in biodegradation (Kirk and Farrell, 1987)13. Variation in the change of pH by different species could be due to various factors like release of enzymes by the fungus to change the pH.
Results revealed that P. varioti reduced comparatively larger amounts of BOD followed by mixed culture. According to Gaddad and Rodgi (1985) BOD removal efficiencies of the filamentous A.niger is poor which contradicts the present investigations where in A.niger exhibits good biodegrading capability. This difference may be due to difference in source of effluents or may be due to difference in strains of microbes. Further they suggested that fungi in combination show better BOD removal efficiency than individual forms.
COD was reduced effectively by P.varioti, P.chrysosporium and A.niger and also by mixed culture. Within the four microbes P.varioti showed higher reduction of COD. This reduction of COD many depend on the growth medium. Use of Actinomycetes strains for effective reduction of cod from tannery effluent was reported by More et al. (2001)14. It was evident from the above results that P. varioti and mixed culture are able to degrade the TSS above 80% when compared to the other groups on the 12th day. It is interesting to note that degradation of TDS by different Fungi was not appreciable, but the mixed culture was able to reduce at least 53.66% in 12 days.
Studies of Blanchette (1991)15, Panneerselvan (1998)16, Prabakar (1999)17 shows that P.chrysosporium is able to degrade BOD, COD, TDS and TSS 90% to 95%, while P.varioti and A.niger has shown better biodegradation capability. Whatever be the fungal species maximum biodegradation was not very effective at higher concentration of effluent, which could be due to the high organic and inorganic load in the tannery effluent. According to Goel (2000)18, single microbe do not have all the enzymes to degrade different kinds of organic matter, therefore a mixture of microbes of various kind is required to completely degrade the organic matter which justifies the present investigation.
Hence it may be suggested that instead of using monoculture of fungus, co-culture/mixed cultures with immobilization will be beneficial for biodegradation and purification of effluents.
Conclusion
Eighteen different species of fungi were identified from the tannery effluents collected Chennai. Out of these four dominant species of fungi like A. niger, A. terreus, P. varioti and P. chrysosporium were selected for biodegradation experiments. Biodegradation of raw tannery effluent in different day intervals (viz. 6 and 12th day) by individual and mixed cultures was carried out with A. niger, A. terreus, P. varioti and P. chrysosporium. Important physiochemical characters namely pH, BOD, COD, TSS and TDS were analysed in the biotreated tannery effluent
Results of biodegradation of tannery effluent was found to be successful resulting in change of pH from acidic to neutral, reduction of BOD, COD, TSS and TDS by over 50 to 80% thereby satisfying CPCB (1995)19standards for effluent discharge.
The above study indicates that instead of single fungus either co-culture or mixed culture must be used for biodegradation of tannery effluent. Moreover the study reveals the capability and role played by each fungus in the biodegradation process studied.
References
- Alexander, M. Biodegradation of chemicals of environmental concern. Science 232:132-138 (1981).
- Ninnekar, H.Z. Biodegradation of Environmental pollutants, Environ.Biodeg. 149-154 (1992).
- Radha, R. Microbial analysis of tannery effluent. M.Sc. Disseration submitted to the University of Madras, Chennai (1995).
- More, S.V., John, S., Rao, B.S., Nair, B.U., and Laxman, R.S. Chromium removal and reduction in COD of tannery effluents by Actinomycetes. Ind, J. Environ. Health. 43(3):108-113 (2001).
- APHA , Standard methods for the examination of water and wastewater (17th end), American public health association (2005).
- Booth, C. Methods in microbiology .Academic press Network, 4:795 (1971).
- Gilman, J.C. A manual of soil fungi, Oxford and IBH Pub, co. Calcutta (1967).
- Subramanian,C.V. Hyphomycetes (An account of Indian species, except carpospores) ICAR, New Delhi (1971).
- Ellis, M.B., Diatomaceous Hyphomycetes, common wealth mycologists institute, Kew, surrey, England (1971).
- Barnet, H.L., and Hunter, B.B. Illustrated genera of imperfect fungi .III edition, Burgers publishing company, Minneapoils, Minnesota (1972).
- Kirk, T.W., Conners, W.J. and Zikus, J.G.. Recommend for a growth substrate during lignin decomposition by two wood rotting fungi. Appl. Environ.Micro.32: 192-194 (1976).
- Rao, V., and Rao, V. Microfungi their diversity and distribution in polluted and non-polluted water bodies from an industrial area (Patan Cheru) in Hyderabad, Andhra Pradesh, India, J. Aqua-Biol. 15 (112): 112-114 (2000).
- Kirk, T.K., and Farell,R.L. Enzymatic “Combustion”. The microbial degradation of lignin, Ann.Rev. Microbiol.41:465-505 (1987).
- Gaddad, S.M., and Rodgi, S.S., Comparative BOD removal efficiency of certain microorganisms isolated from a stabilization pond. Current Science.54 (15) : 749-750 (1985).
- Balnchette,R.A. Delingnification by wood–decay fungi. Ann. Rev. Phytopatho. l.29:381-398 (1991).
- Panneerselvam, A. Studies on sago industry effluent and its bioremediation using the white rot fungus Phanerochaete chrysosporium (Burds). Ph.D.Thesis, University of Madras (1998).
- Prabakar, K.. Studies on Bioremediation of sugar and distillery effluent, Ph.D. Thesis, University of Madras (1999).
- Goel, P.K. Water pollution causes, effects and control, New Age International (p) Ltd., Publ. New Delhi 269 (2000).
- CPCB, Status report of ground water quality in problem areas, Central pollution control board, Delhi (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.





