How to Cite | Publication History | PlumX Article Matrix
In vitro Antibacterial Activity of Organic Solvent Seed Coat Extracts of Borassus flabellifer Linn
G. R. Duddukuri*, Y. N. Sastry, D. S. V. G. K. Kaladhar, K. Kamalakara Rao and K. Krishna Chaitanya
Department of Biochemistry and Bioinformatics, GITAM University, Visakhapatnam - 530 045 India. Corresponding Auhtor E-Mail: dgrao1@gmail.com
ABSTRACT: The in vitro antibacterial activity of organic solvent extracts of Borassus flabellifer L. (Arecaceae) seed coat (soft outer shell) was studied by agar well diffusion method. The tender seed coat of the plant Borassus flabellifer was extracted with different organic solvents viz. petroleum ether, acetone, ethyl acetate, ethanol and methanol. The effect of antibacterial potential was examined against Gram positive bacteria i.e., Staphylococcus aureus, Bacillus subtilis and Gram negative bacteria i.e., Escherichia coli, Klebsiella pneumoniae. Among the different organic solvent extracts of the seed coat tested, methanol extract has showed consistently significant inhibitory activity on different bacterial species. Furthermore, the minimum inhibitory concentration studies carried out by broth dilution assay and found the MIC ranged between 3.12 to 6.25 mg/ml.
KEYWORDS: Borassus flabellifer; Seed coat; Antibacterial assay; MIC
Download this article as:Introduction
Naturally occurring antibacterial compounds can be derived from plants, animal tissues, or microorganisms1. Due to the side effects of the present day antimicrobial compounds and emerging antibiotic resistance, the need for developing the newer antimicrobial compounds has been gaining momentum. The ethnomedicinal plants have been screened for newer compounds with potent activity2 all over the world.
It has been reported that the ethanol and methanol extracts of Aloe vera gel showed higher activity while acetone extract, showed least or no activity against most of the tested pathogens3. The antibacterial activity of aqueous and organic extracts of Thymus capitatus L. (Lamiaceae) leaves and stems4. Organic solvent leaf extracts of Eucalyptus have great potential as antimicrobial agents in the treatment of infectious organisms5. The antimicrobial activity of diethyl ether extract of Cassia auriculata and Emblica fischeri showed better promising results in controlling the bacterial growth6. Albizia lebbeck, Cleistanthus collinus, Emblica officinalis, Eucalyptus deglupta, Eupatorium odoratum, Oxalis corniculata, Hevea brasiliensis, and Lantana camara showed profound antimicrobial activity and even active against multi-drug resistant Escherichia coli and Staphylococcus aureus7. Five medicinal plant species, Abrus precatorius (Fabaceae), Amaranthus spinosus (Amaranthaceae), Argyeria nervosa (Convolvulaceae), Vernonia cinerea (Asteraceae), Zizyphus nummularia (Rhamnaceae) were screened for potential of antibacterial activity against four medically important human pathogens8. The petroleum ether extract of Digera muricata (L.) Mart. (Amaranthaceae) showed inhibition against V. cholerae9. The butanolic extract of Cyanodon dactylon was more active against most of the organisms tested10. Methanol extract of Medicago sativa showed significant inhibitory activity against all the tested bacteria followed by chloroform and ethanol extracts11.
The different parts of the Borassus flabellifer are being used for medicinal properties viz. Male flowers are used for anti-inflammatory activity12,13, the juice from flowering stalks used for diabetes14. Oral feeding of mice with palmyrah flour induced the generation of T suppressor cells which were able to suppress the DTH response to SRBC15. The plant has been used in folklore. For example, decoction used for gonorrhea and respiratory ailments, roots, leaves and flowering stalks for restorative, antihelmenthic and diuretic properties, leaf juice used for hiccups, gastric ailments, the sap is laxative16.
Reports are not available on antimicrobial activity of the seed coat. Therefore, the present study has been undertaken to investigate the antimicrobial activity against Gram negative and Gram positive bacteria and selected fungal species.
Material and Methods
Plant material and preparation of plant extract
Borassus flabellifer tender seeds locally termed as ‘Thati munjelu’ were obtained from local market in the summer season from Visakhapatnam, Andhra Pradesh.
Tender seed coat of Borassus flabellifer is removed and air dried then ground into powder which (1g/10ml) was dissolved in different organic solvents like petroleum ether, acetone, ethyl acetate, ethanol and methanol. These were placed in orbital shaking incubator for 3 days and then centrifuged to remove all the debris. Finally supernatant was collected and tested for antibacterial activity.
Microorganisms
The following bacterial strains were used in this study. Gram positive Bacillus subtilis (NCIM2063) and Staphylococcus aureus (NCIM3021), and Gram negative Escherichia coli (NCIM2066), Klebsiella pneumonia (NCIM2957), Proteus vulgaris (NCIM2027) were obtained from National Chemical Laboratory (NCL), Pune.
Antibacterial activity by agar well diffusion method
The bacteria were grown in Muller-Hinton media (HiMedia Pvt. Ltd., Mumbai, India) at 370C and maintained on nutrient agar slants at 40C and stored at -200C. Inoculum of test organisms was prepared by growing pure isolate in nutrient broth at 370C for overnight. The overnight broth cultures was sub-cultured in fresh nutrient broth and grown for 3hrs to obtain log phase culture. The agar plates were prepared by pour plate method using 20ml M-H medium. The sterile M-H agar medium is cooled to 450C and mixed thoroughly with 1ml of growth culture of concerned test organism (1 x 108 cells) and then poured into the sterile petri dishes and allowed to solidify. Wells of 6 mm size were made with sterile cork borer and test extracts were added. The agar plates were incubated at 370C for 24hrs. The diameter of zones of inhibition was measured in mm using HiMedia zone reader17.
Determination the MIC of the methanol extract by broth dilution assay
The minimum inhibitory concentration of the methanol extract was determined using broth dilution assay18. The medium containing different concentrations of plant extract viz., 100, 50, 25, 12.5, 6.25, 3.12 1.56 and 0.78 mg/ml prepared by serial dilution. After inoculation, the tubes were incubated for 24 hours at 37oC. The MIC of each sample was determined by measuring the optical density in the spectrophotometer at 620 nm and comparing the result with those of the non-inoculated broth.
Results and Discussion
The antibacterial activity of different solvent extracts of Borassus flabellifer seed coat was determined against B. subtilis and S.aureus and E. coli, K. pneumonia, , S. marscence. Among the solvent extracts tested, methanol extract has showed consistently significant activity. The significance of antibacterial activity is assessed by determining minimum inhibitory concentration (MIC) required to inhibit the bacterial growth.
Inhibitory effect of different solvent extracts of Borassus flabellifer seed coat on Gram positive bacteria.
As shown in Fig 1., the antibacterial activity of ethyl acetate extract was found to be very significant for B. subtilis as the highest zone of inhibition was observed while methanol extract exhibited significant antibacterial activity consistently on B. subtilis and S. aureus.
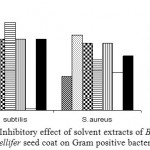 |
Figure 1: Inhibitory effect of solvent extracts of Borassus flabellifer seed coat on Gram positive bacteria.
|
Inhibitory effect of different solvent extracts of Borassus flabellifer seed coat on Gram negative bacteria
The seed coat of Borassus flabellifer exhibited inhibitory activity against Gram negative bacteria such as E. coli, S. marcescence, K. pneumonia (Fig 2.) tested. Moreover, the significant antibacterial activity was found in methanolic extract as compared to other solvent extracts. However, the inhibitory activity is not as significant as with Gram positive bacteria.
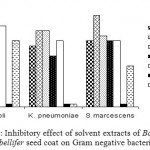 |
Figure 2: Inhibitory effect of solvent extracts of Borassus flabellifer seed coat on Gram negative bacteria.
|
Comparative inhibitory activity of methanol extract of tender seed coat of Borassus flabellifer on different bacterial species
The methanol extract of seed coat of Borassus flabellifer exhibited significant inhibitory activity against all the bacterial species employed. However, the highest zone of inhibition was obtained with S. aureus and S. marcescens followed by with E. coli and B. subtilis. The lowest zone of inhibition was obtained with K. pneumoniae (Fig 3.)
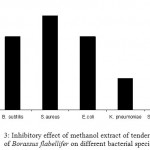 |
Figure 3: Inhibitory effect of methanol extract of tender seed coat of Borassus flabellifer on different bacterial species
|
Determination of Minimum Inhibitory Concentration (MIC)
Minimum inhibitory concentration of methanol extract of seed coat was determined against different Gram positive bacteria like S. aureus and B. subtilis and Gram negative bacterial species like K. pneumonia, E. coli and S. marcescence. As shown in Table 1., the minimum concentration of extract required to inhibit the bacterial growth varies between 3.12 and 6.25 mg crude methanol extract against. The lowest MIC was observed with 3.12 mg/ml S. aureus, while for B. subtilis, K. pneumonia, E. coli and S. marcescence, the MIC was found to be 6.25mg (Table 1.). This observation implies that the presence of potent antibacterial compounds in methanol extract of B. flabellifer.
Table 1: Minimum inhibitory concentration (MIC) of methanol extract of tender seed coat of Borassus flabellifer for antibacterial activity
| S.No. | Bacterial species | MIC (mg/ml) |
| 1 | B.subtilis | 6.25 |
| 2 | S. aureus | 3.12 |
| 3 | E.coli | 6.25 |
| 4 | K.Pneumoniae | 6.25 |
| 5 | S. marcescens | 6.25 |
Conclusion
The above results confirm that the seed coat of Borassus flabellifer exhibit antibacterial activity. Among the different organic extracts tested, crude methanol extract found to exhibit consistent antibacterial activity with relatively lower MIC values indicating to undertake further fractionation analysis to isolate the antibacterial compound of therapeutic importance.
Acknowledgement
The authors acknowledged the support from Department of Biochemistry, GITAM University for providing the necessary research facilities.
References
- Jordon M.C. and David J.N. Natural product drug discovery in the next millennium, Pharmaceut Biol, 139:8-17 (2001).
- Cordell G.A. New roots for an old science, in Atta-ur-Rahman and Basha F.Z., (Eds) Studies in Natural Products Chemistry. Pharmacognosy 13: Bioactive Natural Products (Part A), Elsevier, Amsterdam (1993).
- Rubina Lawrence, Priyanka Tripathi and Ebenezer Jeyakumar. Isolation, purification and evaluation of antibacterial agents from Aloe vera, Brazilian Journal of Microbiology, 40: 906-915 (2009).
- Haitham N.G., Muayad M.A., Khaled M.K., Khaled A.T., and Osama Y.A. Antibacterial activity in vitro of thymus capitatus from Jordan, Pak. J. Pharm. Sci., 22(3): 247-251 (2009).
- Mumtaz J., Mohiuddin K.W. and Fehmeeda K. Studies on antibacterial property of Eucalyptus – The aromatic plant, International Journal of Pharmaceutical Sciences Review and Research, 7 (2): (2011).
- Sekar J. Antibacterial activity of plant extracts – cassia Auriculata and Emblica fischeri, Plant Archives, 10 (2): 819-824 (2010).
- Maji S., Dandapat P., Ojha D., Maity C., Halder S.K., Das Mohapatra P.K., Pathak T.K., Pati B.R., SamantaA A., and Mondal K.C. In vitro antimicrobial potentialities of different Solvent extracts of ethnomedicinal plants against clinically isolated human pathogens, Journal of Phytology, 2(4): 57–64 (2010).
- Ashok K.J. and Sujata G. Antibacterial Potential of some medicinal plants, International Journal of Biological Technology, 2(1):4-6 (2011).
- Pratima M. and Sundar S.M. Phytochemical and Antimicrobial activity of Digera Muricata (L.) Mart, e-Journal of Chemistry, 7(1): 275-280 (2010).
- Yogesh C., Hardik R.M. and Vamshikrishna B.A. Antibacterial activity of Cyanodon dactylon on different bacterial pathogens isolated from clinical samples, IJPSR, 2: 16-20 (2011).
- Doss A., Parivuguna V., Vijayasanthi M. and Sruthi S. Antibacterial evaluation and phytochemical analysis of Medicago sativa L. against some microbial pathogens, Indian Journal of Science and Technology, 4(5): (2011).
- Mahesh S.P. Evaluation of anti-inflammatory activity of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) in experimental animals, Journal of Medicinal Plants Research, 3(2): 049-054 (2009).
- Mahesh S.P., Patil M.B., Ravi K. and Sachin R.P. Evaluation of anti-inflammatory activity of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) in experimental animals, Journal of Medicinal Plants Research, 3(2): 049-054 (2009).
- Masayuki Y. Medicinal flowers. XII.(1)) New Spirostane-Type Steroid Saponins with Antidiabetogenic Activity from Borassus flabellifer, Chem Pharm Bull, 55(2): 308-316 (2007).
- Shamala D., Arseculeratne S.N., Pathmanathan R., McKenzie I.F.C. and Pang T. Suppression of cell-mediated immunity following oral feeding of mice with palmyrah (borassus flabellifer l) flour, Australian Journal of Experimental Biology and Medical Science 63: 371–379 (1985).
- Atchley, A.A. Nutritional value of palms. Principes, 28(3):138-143 (1984).
- Kaladhar D.S.V.G.K. and Siva Kishore N. Antimicrobial studies, biochemical and image analysis in Mirabilis jalapa, International Journal of Pharmacy &Technology, 2(3): 683-693 (2010).
- Andrews J.M. Determination of minimum inhibitory concentrations, J Antimicrob Chemother, 48 (1): 5-16 (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.





