Manuscript accepted on : June 05, 2011
Published online on: 28-06-2011
In vitro Antifungal Studies of Novel Synthetic Compounds against Alternaria brassicae
Vinod Kumar Sewariya1, Richa Shrivastava1, Gbks Prasad2 and Kishor Arora3
1Depatment of Botany and Microbiology, Govt. KRG PG Auto College Gwalior India.
2Depatment of Biotechnology SOS, Jiwaji University, Gwalior (India).3Depatment of chemistry, Govt. KRG PG Auto College Gwalior India.
Corresponding Author E-mail:kishorarora@reddifmail.com
ABSTRACT: A total of seven Pyrazolones and Pyridines and three Schiff bases were evaluated in-vitro against Alternaria brassicae Causative organism of black leaf spot disease among crucifers. Pyrazolone, Pyridine and Schiff bases (C1-C10) are tested in-vitro. All ten novel synthetic compounds designated C1 to C10 did not show antifungal activity against Alternaria brassicae.
KEYWORDS: Alternaria brassicae; leaf spot; Pyrazolones; Pyridines and Schiff base
Download this article as:| Copy the following to cite this article: Sewariya V. K, Shrivastava R, Prasad G, Arora K. In vitro Antifungal Studies of Novel Synthetic Compounds against Alternaria brassicae. Biosci Biotech Res Asia 2011;8(1) |
| Copy the following to cite this URL: Sewariya V. K, Shrivastava R, Prasad G, Arora K. In vitro Antifungal Studies of Novel Synthetic Compounds against Alternaria brassicae. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9333 |
Introduction
Plant diseases are known since ancient times and fossil evidence indicates that plants were affected by diseases 250 million years ago. There are many examples of plant diseases that have made impact on society and have been changed human history [1]. Loss of crops from plant diseases may results in hunger and starvation, especially in less developed countries where access to disease control method is limited and annual losses of 30 to 50% are common for major crops like mustard in country like India.
Mustard (Brassica campestris) comes under the family Cruciferae (Brassicaceae). Mustard as well as other crucifers is affected by different species of fungal phytopathogens. Different species of pathogens of Alternaria family are responsible for black leaf spot disease infection in four species of crucifers. viz; 1. Brassica campestris (Mustard) infected by Alternaria brassicae, and Alternaria brassicicola 2. Brassica oleraceae (Patta gobhi) infected by Alternaria brassicae, Alternaria brassicicola and Alternaria raphani 3. Brassica nigra (Black mustard / kali rai) infected by Alternaria brassicae, Alternaria brassicicola and 4. Raphanus sativus (Raddish) infected by Alternaria brassicae, Alternaria brassicicola and Alternaria raphani [2].
Fungal plant diseases are one of the major concerns to agricultural production. It has been estimated that total losses as consequence of plant diseases reach 25% of the yield in Western countries and almost 50% in developing countries. Of this, one third is due to fungal infection (Bowyer 1999). So there is a pressing need to control fungal diseases. [3]
The necrotrophic nature of Alternaria sp. typically leads to extensive damage of the plant and harvest product with seedling seldom surviving an attack [4-6].
Alternaria brassicae are cosmopolitan in their distribution but occur sporadically. The pathogen are greatly influenced by weather with the highest disease incidence reported in wet season and in area with relatively high rainfall. Alternaria brassicae can affect host species at all stages of growth, including seeds. Alternaria symptoms often occurs on the older leaves, since they are closer to the soil and more readily infected as a consequences of rain splash or wind blown rains. Alternaria brassicae remain viable for a long period of times as spores on seed coat or as mycelium in seed as well as in infected plant debris [7,10,11].
Several synthetic as well as natural compounds were screened against these microbial diseases by several workers. Synthetic compounds available in market to control fungal phytopathogenic diseases include- Biltox, Mancozeb (Dithane M-45), Maneb and Thiram for Alternaria brassicae, Alternaria brassicicoloa, (Black or brown spot disease in Mustard and crucifers)[8].
In the present paper, ten novel synthetic organic compounds were screened in-vitro against Alternaria barssicae. The selection of ten novel synthetic organic compounds was based on the fact that none of these compounds were not previously screened against these selected phytopathogens [9,12.13].
The characterization, establishment, computational studies and Structure activity relationship (SAR) of selected ten novel synthetic organic compounds also reported in this paper. The whole in-vitro antifungal study was focused to find out the solution for pest management of phytopathogen – Alternaria barssicae on local area crops of crucifers including Mustard.
Material and Method
Fungal Culture
Plant pathogen Alternaria brassicae (ITCC-5097) were procured from ITCC, division of plant pathology, I.A.R.I. New Delhi. The organism was cultured on Potato carrot agar (PCA) and Potato carrot broth (PCB) and incubated at 22-24 ºC for 5-7 days for revival and sub culturing of test organism. For inoculation 100 µl. of actively growing cells of (OD 106–108 / ml.) Alternaria brassicae inoculated Potato carrot broth (PCB) was used to inoculate sterile Potato dextrose agar plates through spread plate swab method.
Synthetic compounds
The ten novel synthetic compounds of three groups viz: Pyrazolones, Pyridines and Schiff bases (C1-C10) as listed out in Table 1 synthesized in the laboratory and their structures were confirmed by standard analytical techniques. The Structures and some physical properties of Pyrazolones, Pyridines and Schiff bases compounds are listed out in Table.1
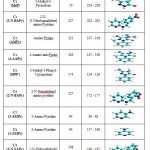 |
Table 1: Structures and some physical properties of Pyrazolones, Pyridines and Schiff bases compounds are listed below.
|
Preparation of inoculum
After 5-7 days of incubation at 22-24 ºc, the test organism viz; Alternaria brassicae, was revived. A loopful of culture was taken and dispensed in 50 ml. of Potato carrot broth (PCB) in a conical flask and kept at 22-24 ºc for further 5-7 days for the preparation of inoculum.
Preparation of Petri plates and media
Potato Carrot Agar (PCA), Potato Carrot Broth (PCB), was used. Media were autoclaved at 121ºc for 15 lbs pressure for 15 minutes. Media were cooled and then plates were prepared by dispensing 15-20 ml. media per plate. Plates were kept in the same position for ½ hour for solidify media and kept inverted in the incubator at 22-24 ºc over night for sterility checking.
Preparation of antimicrobial discs
Whatman 1 filter paper discs of 6 mm. diameter were punched out from barge sheet and were autoclaved at 121ºC, at 15 lbs for 15 minute [14].
Preparation of serial dilutions of test compounds
Each compound was serially diluted (50mg.→05mg.) in DMF as shown in Table 2 and each dilution of each compound was tested against Fungal pathogen.
Inoculation and Incubation
100 µl of actively growing cells of (OD 106-108/ml.) Alternaria brassicae ITCC-5097 was used to inoculate sterile Potato Carrot Agar (PCA) plates by spread plate swab method. The whatman paper discs were dispensed on glass plates and each was load with 5µl. volume of pre designated dilution C1→C10 as shown in Table 2. The discs were left air dried in the laminar air flow and were then carefully transferred to inoculated plates at pre designated positions. The plates were then incubated at 22-24 ºC for 5-7 days [15-17,18].
Table 2: Concentrations of the test compounds used in the study.
| Dilutions
|
Test tube No. | Concentration of Compound |
| D0 | 1 | Negative control |
| D1 | 2 | 5 mg. |
| D2 | 3 | 10 mg. |
| D3 | 4 | 15 mg. |
| D4 | 5 | 20 mg. |
| D5 | 6 | 25 mg. |
| D6 | 7 | 30 mg. |
| D7 | 8 | 35 mg. |
| D8 | 9 | 40 mg. |
| D9 | 10 | 45 mg. |
| D10 | 11 | 50 mg. |
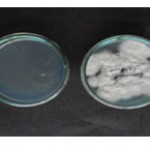 |
Figure 1: Alternaria brassicae (ITCC-5097) in Petri plates and slants.
|
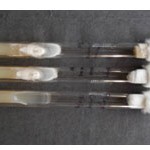 |
Figure 2: Alternaria brassicae (ITCC-5097) in Petri plates and slants.
|
Result and Discussion
Antifungal activity of Pyrazolones and Pyridines
A total of seven Pyrazolones and Pyridines viz; 3-Methyl-5-Pyrazolone (C1), Amino Pyrine (C3), 3-methyl-1-Phenyl-5-Pyrazolone (C5), 3-Amino Pyridine (C7), 4-Amino Pyridine (C8), 4-Amino anti-pyrine (C4) and 2-Benzyl Pyridine (C10) were tested for anti-fungal activity against Alternaria brassicae, All seven Pyrazolones and Pyridines designated C1, C3, C5, C7, C8, C4, and C10 did not show antifungal activity against Alternaria brassicae. (Fig 3-9)
 |
Figure 3: Antimicrobial Activity of Compound C1 against Alternaria brassicae.
|
 |
Figure 4: Antimicrobial Activity of Compound C3 against Alternaria brassicae.
|
 |
Figure 5: Antimicrobial Activity of Compound C5 against Alternaria brassicae.
|
 |
Figure 6: Antimicrobial Activity of Compound C7 against Alternaria brassicae.
|
 |
Figure 7: Antimicrobial Activity of Compound C8 against Alternaria brassicae.
|
 |
Figure 8: Antimicrobial Activity of Compound C4 against Alternaria brassicae.
|
Antifungal activity of Schiff bases:
A total of three Schiff bases viz; 2-N-[3-Nitrobanzalidene] amino Pyridine (C2), 2-N-[benzalidene] amino pyridine (C6) and 2-N-[Salysaldehyde] amino pyridine (C9) were tested for anti-fungal activity against Alternaria brassicae.None of three Schiff bases described above did not show antifungal activity against Alternaria brassicae. (Fig 10-12)
 |
Figure 9: Antimicrobial Activity of Compound C10 against Alternaria brassicae.
|
 |
Figure 10: Antimicrobial Activity of Compound C2 against Alternaria brassicae.
|
 |
Figure 11: Antimicrobial Activity of Compound C6 against Alternaria brassicae.
|
 |
Figure 12: Antimicrobial Activity of Compound C9 against Alternaria brassicae.
|
The present in-vitro antifungal investigation clearly demonstrates the did not show antifungal activity against Alternaria brassicae. The results of in-vitro antifungal study indicate a total of seven Pyrazolones and pyridines, a total of three Schiff bases are not useful compounds in management of soil born fungal diseases caused by Alternaria brassicae, since phytopathogenic fungi cause diseases in many important Crucifers crop plants including Mustard.
There is no study available in literature, to the best of our knowledge, on these synthetic Compounds with anti-fungal activity against Alternaria brassicae. This is the first study evaluating the anti-fungal activity of Pyrazolones, Pyridines and Schiff bases Compounds.
Analysis and Charactrization of Compouunds
Analytical Studies of Compounds
Establishment of these compounds was carried out on some physicochemical studies viz: melting point (M.pt.) determination, CHN analysis spectral studies including IR, Mass and NMR spectral studies [19, 20]. The preliminary investigation of the compounds viz: m. pt. / b. pt. determination, elemental analysis viz: CHN analysis of Compounds noted on usual methods in Chemistry Research Laboratory, Department of Chemistry, Govt. K.R.G. P.G. Autonomous college Gwalior M.P. CHN analysis of these compounds were carried out on Elemental analyzer Elemental Vario EL III, IR – Perkin Elmer Infrared Spectrophotometer in the range 4000 to 50m-¹, Mass – Mass Spectrometers Jeol SX-102 (FAB) and NMR spectral studies recorded on NMR Spectrometer Bruker DRX-300 at SAIF CDRI Lucknow. [21-23].
Mass spectral studies of the Compounds
Mass spectral studies of the compounds help in establishing the compounds by means of into fragmentation studies [24]. Apart from it studies of parent ion peak in mass spectra of any compound help in the establishment of into molecular weight [25, 26].
Mass spectral studies of the compounds chosen for this study show that the parent ion peaks in the spectra of these compounds appear at the m/e values where these are expected to come. The parent ions peaks for C1, C2, C3, C4, C5, C6, C7, C8, C9, C10 appears at ca 98, 231, 203, 174, 95 , 95, 170, 228, 228, 199. This confirms the molecular weights of the compounds C1, C2, C3, C4, C5, C6, C7, C8, C9, & C10 as compared to their formula weights [27-28].
Infrared spectral studies
Infrared absorption studies of Pyrazolones have been assigned by a comparison of these spectra with those of Pyrazole, five membered ring systems [29]; the mono-substituted benzene ring system [30] and with those already reported. [31,32,36].
The C=O stretching frequencies occur at 1614.3, 1661, 1665 & 1598 respectively in case of MeP, AmPy, 4-AAPy & MPP [27, 34]. The strong band at ca has been assigned to the ring stretching of 5 membered ring in Pyrazolone compounds five membered ring hetero atomic compounds are found to have two strong bands near 1590-1560 and 1450-1430 cm-¹ Which are considered to be characteristic of five membered ring [35, 36]. The bands assigned to the benzene several other absorptions associated with C-H out of plane deformation modes appear in the region 980-840 Cm-¹ in Pyrazolones [23, 37, & 38].
Infrared absorption of all Schiff bases have been assigned by comparison of their spectra with those of the five membered Pyrazole ring system [29], the mono substituted benzene ring system [30], and substituted anti-pyrines [32,33,37,39,40]. The notable peaks in case of Schiff base appear at ca 1604-1598 Cm-1 which are attributed to (C=N-) the azomethenic group frequencies. All other diagnostic peaks such as ring stretching, N- phenyl stretching ע (C-N), ring breathing of benzene, C-N-C bending and other deformation peaks matched well with the previous observations [41-43].
Computational Studies of Compounds:
The most common and popular Computational (Semi-empirical) methods used today in the field of Chemi-informatics are MNDO, ZINDO, MNDO/3 AM1 and PM3 etc. In this paper we wish to report AM1, PM3, MNDO and ZINDO calculations for compounds under study. The AM1, PM3, MNDO and ZINDO, Hyperchem 8.0 software package were used to calculate the structure activity relationship (SAR) related parameters. All these calculations were carried out on Pentium core-2 Duo machine configuration with windows-Microsoft windows XP [44-46]. In present study no significant SAR equation were obtained.
References
- Vipul Gohel, Anil Singh, Maisuria Vimal, Phadnis Ashwini and Chhatpar H.S. “Bioprospecting and antifungal potential of chinolytic microorganisms” African Journal of Biotechnology, 5 (2), 54-72, (2006).
- Christopher B Lawrence, Thomas K Mitchell, Kelly D. Yangrae, Cho Robert A, Cramer Jr. and Kwang-Hyung Kim “At Death’s Door Alternaria Pathogenicity Mechanism” Plant Pathol. J., 24 (2), 101-111, (2008).
- Levetin and Mc Mahon, “Plant and society” IIIrd-Edition Tata Mc Graw-Hills, (2003).
- JS Chouhan, Anoop Badoni, N. Indrakumar Singh and Seema Ali “Effects of Alternaria on some members of family Brassicaceae of Gahrwal Himalaya” New York science Journal 2 (6), 80-85, (2009).
- Singh SN, Goswami GP “Management of sugarcane wilt disease caused by Fusarium moniliforme through chemical and physical set treatment” College of agriculture, Jawaharlal Nehru Agriculture university sehore M.P. (2006).
- Nanda Kumar Rajendran, Jayapradha Ramakrishnan “In-vitro evaluation of antimicrobial activity of crude extracts of medicinal plants against multi-drug resistant pathogen” Biyoloji Bilimler Arasturma Dergist, 2 (2), 97-101, (2009).
- Arik Makovitzki, Ada Viterto, Yariv Brotman, Ilan Chet, and Yechiel Shai “Inhibition of fungal and bacterial plant pathogens in-vitro and in planta with ultra short cationic lipopeptiedes” Applied and environmental microbiology, 73 (20), 6629-6636, (2007).
- V. Wangeningen, Jurgen Kohl & Jan van der Wolf, “Alternaria brassicicola and Xanthomonas campestris pv. campestris in organic seed production of Brassicae; Epidemiology and seed infection” Plant Research International, 363, 01-28, (2005).
- Humpherson-Jones, F.M., “Survival of Alternaria brassicae and Alternaria brassicicola on crop debries of oilseed rape and cabbage” Appl. Biol., 115, 45-50 , (1989) .
- Humpherson-Jones, FM & K Phelps, “Climatic factors influencing spore production in Alternaria brassicae and Alternaria brassicicola” Annals of Applied Biology, 114, 449-458 , (1989).
- Chen, L.Y. & T.V. Price “Dark leaf spot (Alternaria brassicicola) on Chinese cabbage: temporal spread and its influencing factors” Australian Journal of Agricultural Research, 53, 1095-1103, (2002).
- Gurdip Singh, Om Prakash Singh, G.P. Rao and S.R. Sharma, “The Vapour Action of Essential Oils and Monoterpenoids against Pathogenic Fungi” Sugar Tech, 4 (1 & 2), 69-71, (2002).
- Park, Cheol Nam, Jung Min Lee, Dongho Lee and Beom Seok Kim “Antifungal Activity of Valinomycin, a Peptide Antibiotic Produced by Streptomyces sp. Strain M10 Antagonastic to Botrytis cierea” Microbiol. Biotechnol, 18 (5), 880-884, (2008).
- Maurizio Del Poeta, Wiley A. Schell, and John R. Perfect, “In-vitro Antifungal Activity of Pneumocandin L-743, 872 against a variety of clinically important molds” Antimicrobial Agents and Chemotherapy, 41 (8), 1835-1836, (1997).
- Dong-Sun Jung, Yeo-Jung NA, and Ki Hyun Ryu “Phyolgenic Analysis of Alternaria brassicicola Producing Bioactive Metabolites” The Journal of Microbiology, 40 (4), 289-294, (2002).
- Babu Joseph, Muzafar Ahmad Dar and Vinod Kumar “Bio efficacy of Plant Extracts to Control Fusarium solani sp. Melongenae incitant of Brinjal wilt” Global Journal of Biotechnology & Biochemistry, 3 (2), 56-59, (2008).
- Abdul Rashid and Narendra Singh “Role of Plant Parasitic Nematodes on the Incidence and Severity of Sugarcane Wilt” Sugar Tech, 4 (1 & 2), 61-62, (2002).
- Bauer AW, Kirby WM, Sherris JC, Turck M, “Antibiotic Susceptibility tests by a standardized single disk method” J. Clin. Pathol. 45, 493-496, (1966).
- Kishor Arora “Physico-Chemical investigations of some high coordination compounds of thorium (iv) and dioxouranium (vi) with some oxygen donor ligands” D. Thesis submitted to Meerut University Meerut (1992).
- K. Agarwal, P.C. Jain, V. Kapur & T.N. Srivastava, “Thorium (iv) complexes of mono-N-oxide of 2-2’-bipyridine and 1, 10- phenon throline” Transition Met Chem, 5, 237, (1980).
- K. Agarwal, Kishor Arora, Miss Priyanka & I. Charovati, Polish J. “Some high coordination compounds of thorium (iv) and dioxyuranium (vi) with Schiff bases derived from 4-amino antipyrine” Chem, 67, 1913, (1993).
- K. Agarwal, Kishor Arora, & Prashant Dutt, “Some high coordination compounds of thorium (iv) and dioxyuranium (vi) derived from hydrazones of isonicotinic acid hydrazides” Polyhedron 13, 957, (1994).
- Kishor Arora, K. P. Sharma & A.R. Khan; “Studies of thorium (iv) and dioxyuranium (vi) metal complexes of a Schiff base ligands” J .Saudi Chem Soc. 8, 241, (2004).
- M. Silverstein, C.A. Basaler and T.C. Morrell, “Spectrophotometric Identification of organic compounds” VIth Edition, John wiley & Sons, (1997).
- S. Kalsi, “Spectroscopy of organic compounds” Vth Edition, New Age International (P) Ltd. (2002).
- Irving Sunshine “Handbook of Spectrophotometeric data of drugs” CRC Press (2002).
- K. Agarwal and Kishor Arora; “Synthesis and characterization of thorium (iv) adducts of some pyrazolone ligands” Synth. React inorganic & Met. Org. Chem. 23, 653, (1993).
- R. Khan, Kishor Arora and K.P. Sharma, “Studies of thorium (iv) and dioxyuranium (vi) complexes of a Schiff base ligands: part-2” Asian J. Chem. 16, 353, (2004).
- Ram K Agarwal, Himanshu Agarwal, “The synthesis structure and bonding in some Lanthanide (iii) coordination compounds of 4-[(N-Furfural) Amino] Antipyrine” Synthesis and Reactivity in inorganic, Metal organic & nano metal and chemistry, 31 (2), 263-276, (1994).
- R. Bellamy “Infrared spectra of complex compounds” Mathuen London (1964).
- N. Sathyanarayan and C.C. Patel, “Oxalato complexes of oxovanadium (iv)” J. Inorg. Nucl. Chem., 28, 2277-2283, (1966).
- K. Agarwal, Kishor Arora and R.K. Sarin “Magneto and Spectral studies of Lanthanide (iii) Nitrate complexes of 4 vinyl pyridine” Synthesis and Reactivity in inorganic, Metal organic & nano metal chemistry, 24 (5), 735-747, (1994.)
- K. Agarwal, A.K. Srivastava and T.N. Srivastava, “Thorium (iv) complexes of pyrazolone ligands” Proc. Nat. Acad. Sci. 51 (44), 79, (1981).
- Agarwal R.K., Kishor arora, Priyanka and Chakrovorti I, “Some High coordination compounds of Thorium (iv) and dioxouranium (vi) with Schiff bases derived from 4-Amino antipyrine” Polish Journal of Chem., 67 (11), 1913-1923,(1993).
- K. Agarwal and Kishor Arora; “Synthesis and characterization of dioxyuranium (vi) complexes of Pyrazolone ligands” Polish J. Chem. 67, 25, (1993).
- Ran K. Agarwal and Surendra Prasad “Synthesis, Biological Spectral and Thermal properties of oxovanadium (iv) complexes of N, N, S- containing Legands” Reviews in Inorg. Chemistry, 26 (5), 471-492, (2006).
- Kishor Arora, D.D. Agarwal and Aarti Gupta, “ Studies on Lanthanide (iii) nitrate complexes of Schiff base ligands” International Journal of physical sciences, 17, 297, (2005)
- Aarti gupta, Kishor Arora and D.D. Agarwal, “Synthesis and characterization of Lanthanide (III) perchlorate complexes of some Schiff base ligands” Asian J. Chem. 16, 489, (2004).
- Kishor Arora, K.P. Sharma and A.R. Khan, “Synthesis and studies of thorium (iv) and dioxyuranium (vi) complexes of a Schiff base ligand” Oriental J. Chem. 19, 659, (2003).
- Kishor Arora, K.P. Sharma and Mukesh Sharma, “Lanthanide (III) nitrate complexes of Schiff bases” Journal of Chemistry, 6 (51), 5201-5210, (2009).
- Arora, R.K. Agarwal and G. Singh, “Cumbustion studies and thermal decomposition kinetics of 4-[N(-hydroxy-1-naphthalidene) amino] antipyrine thiosemicarbazone complexes with nickel (ii) and cobalt (II) chloride” Oxidation communications, 18, 150, (1995).
- K. Agarwal, K. Arora and P. Dutt. “Studies of 4-N[(Furfural) amino] antipyrine complexes of thorium (iv) and dioxyuranium (vi)” Proc. Mat. Acad. Sc. 67, II (1997).
- K. Agarwal, K. Arora, Himanshu Agarwal and R.K. Sarin; “Synthesis and structural investigation of thorium (iv) and dioxyuranium (vi) complexes with 4-vinyl pyridine” Chem. 25, 899, (1995).
- B. Lipkowitz and D. B. Boyd, J.J. P Stewart “Reviews in computational chemistry”. Ed. V CH, Vol. 1, 45, (1990).
- J.S. Dewer, E. G. Zoebisch, E.F. Healy and J.J.P. Stewart, “Semi-empirical methods” J. am. Chem. Soc., 107, 3902, (1994).
- Animesh Pathak “Quantum chemical calculations for some complexes” Ind. Chem. Soc., 77, 402, (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.





