Manuscript accepted on : February 15, 2011
Published online on: 28-06-2011
Boby T. Edwin* and Prabha D. Nair
Department of Botany, Thangal Kunju Musliar College of Arts and Science, Karicodue, Kollam Division of Tissue Engineering and Regeneration Technologies, Biomedical Technology Wing, Satelmond Palace, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram India.
Corresponding Author E-mail: bobytedwin2003@gmail.com
ABSTRACT: Hemigraphis alternata (HA) have been traditionally identified in Indian medicine (Ayurveda) to promote the healing of soft tissue wounds and minor injuries. This study is to elucidate its role in wound healing through its effects on fibroblasts and endothelial cells. The effects of aqueous and ethanolic extract of HA (AEHA and EEHA) on proliferation and migration of fibroblast was assayed by MTT and scratch-wound healing assay. Expression of angiogenic markers like tyrosine kinase receptors (KDR, FLT 4), Platelet endothelial cell adhesion molecule (CD31) and vascular endothelial cadherine (VE Cadherin) by endothelial cells when treated with HA were analysed by semiquantitative RT PCR. The study showed that AEHA at a concentration of 2.5mg/ml and 3.5mg/ml revealed an enhancement in proliferation in serum free and serum containing culture conditions respectively while EEHA was toxic to cells. Proliferation of AEHA was associated with a 60% protein fraction which revealed a band of 21.8 kD peptide. The AEHA at a concentration of 180g showed a wound re-epithelialization to 97% in a fibroblast scratch wound model. PCR assay revealed enhanced expression of angiogenic markers like KDR, FLT 4, CD31 and VE Cadherin. These results evaluated HA as a herb with potential implications in wound healing as it is seen to influence fibroblast and endothelial cells, the key players during wound healing.
KEYWORDS: Hemigraphis alternata; wound healing; VE Cadherin; scratch wound healing assay
Download this article as:| Copy the following to cite this article: Edwin B. T, Nair P. D. In Vitro Evaluation of Wound Healing Property of Hemigraphis Alternata (Burm. F) T. Anders Using Fibroblast and Endothelial Cells. Biosci Biotech Res Asia 2011;8(1) |
| Copy the following to cite this URL: Edwin B. T, Nair P. D. In Vitro Evaluation of Wound Healing Property of Hemigraphis Alternata (Burm. F) T. Anders Using Fibroblast and Endothelial Cells. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9316 |
Introduction
Since mediaeval times, plants have played an important role in the life of humans, as a major source of food, as well as for maintenance and improvement of health and for the elimination of enemies (Kumara et al., 2007, Puratchikody et al., 2006.). About 80% of the world’s population still relies on plant-based medicines for their primary health care (WHO 2002-05). Ayurveda an ancient (before 2500 B.C) Indian system of health care and longevity (Dev, 1999) involves considerable use of plant based drugs. Many traditional remedies based on systematic observation and methodologies and time tested for their efficacies are mostly invalidated with scientific evidence. Aqueous paste of Hemigraphis alternata (Blume) H.G. Hallier (Common Name: Red Ivy, Red Flame Ivy) is a well known wound healing agent used in traditional medicine in India. It is one of the exotic plants adapted to India from Malay Archipelago and belongs to family, Acanthaceae Juss. The plant is a prostrate, rooting perennial. Even though the plant is used extensively in wound healing, to the best of our knowledge, only one report is available regarding the wound healing, and anti inflammatory properties of this plant leaf suspension/paste in excision model in mice (Subramoniam et al ., 2001).
Wound healing is a complex and dynamic process of restoring cellular structures and tissue layers. The human adult wound healing process can be divided into 3 distinct phases: the inflammatory phase, the proliferative phase, and the remodeling phase. Within these 3 broad phases is a complex and coordinated series of events that comprise the phenomena of exposure of platelets to collagen, release of clotting factors like cytokines (platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-ß), phagocytosis to remove foreign materials, migration of fibroblasts, angiogenesis and collagen deposition (Lawrence., et al 1994, Kim., et al 1998, Gillitzer., 2001). Fibroblasts and endothelial cells are the major cells involved in the process of wound healing. Endothelial cells play a major role in angiogenesis which require activation of proangiogenic factors. Above mentioned processes are orchestrated in a controlled manner by a variety of growth factors (Ozturk et al., 2004). The culmination of these biological processes results in the replacement of normal skin structures with fibroblastic mediated scar tissue.
Much more insight into the pharmacological functions and mechanisms of Hemigraphis alternata are required with experimental evidences to support its use as a wound healing agent. In this study we attempted to study the effect of Hemigraphis alternata extract on the cell migration and proliferation of fibroblast cells and endothelial cells and selective expression of proangiogenic marker mRNA of endothelial cells in the presence of the plant extract.
Materials and Methods
Plant materials and chemicals
Tissue culture grade Dulbecco’s modified Eagle’s medium – High glucose (DMEM-LG), Dulbecco’s phosphate buffered saline (DPBS), Trypsin-EDTA, Fetal bovine serum (FBS), Foetal calf serum (FCS)- Hyklon and Penstap were purchased from Invitrogen (India & USA). Dimethyl sulphoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) are from sigma (USA). All culture media were supplemented with 1x Penstap and 10% FBS. The molecular biology chemicals were purchased from Fermentas. The cell line L929 was supplied by National Centre for Cell Sciences (NCCS) Pune, India. Umbilical cords were collected from Hospitals with informed consent of the donor. Plant Hemigraphis alternata was collected from different localities of Trivandrum in Kerala, India. The plant material was identified by standard taxonomical methods using flora of Madras. The voucher specimen is preserved as herbarium sheet no .1 in the institute.
Extraction of plant materials
100 g of Hemigraphis plants were surface sterilized with sodium hypochlorite and water sterilized in Millipore water. The washed leaves were ground into a paste using an electric blender with 200 ml of Millipore water, filtered and lyophilized to get brown powder. The extract powder is dissolved in DMEM –HG at the ratio of 50 mg /10 ml. The protein content and carbohydrate content in the extract was quantified by Biuret and Anthron reagent respectively as per the standard protocol (Layne 1957, Morris 1948)
Isolation and characterization of Human Umbilical Vein Endothelial Cells
Human umbilical cords (15-20 cm) were collected and stored for up to 12 h at 4 °C in medium 199 containing penicillin (50 units/ml)/streptomycin (50 mg/ml).Venous endothelial cells were harvested by collagenase digestion as originally described by Jaffe et al.(1973). The cells from each cord were plated out in Nunc 25-cm2 tissue-culture flasks, sub cultured and seeded onto 20 mm x 11 mm glass coverslips All processed involved incubation of cells at 37 °C in an humidified atmosphere of air/CO2 (19:1). Cells on coverslips were processed and immunofluorescently labeled by the sequential antibody staining method. Purified anti-VWF monoclonal was used as primary antibody. Fluorescein isothiocyanate (FITC) conjugated chicken IgG were the secondary antibodies (diluted at 1:200).
Assay of cell proliferation
The dry crude extract powder was redissolved in DMEM with out serum before preparing serial half-log 10 dilution in a 0.15 mg/ml to 5.5 mg/ml range. Proliferation was assayed using tetrazolium dye (MTT) (Mosmann, 1983, Niks, 1990). Fibroblast and endothelial cell monolayers (L929) in culture were trypsinized and washed with culture medium. The cells were plated at 1500 cells/well in 96-well flat-bottomed plate. After a 24 hr preincubation period, extract duplicates were incubated for 24, 48, 72 hr at 37oC in a humidified incubator with 5% CO2, the supernatants were removed from all wells and 25 ml of MTT was added, incubated for 2 hr at 37°C. The absorbance was read at 570 nm on UV-Vis spectrophotometer (Cary 100) .
Hoechst staining
Fibroblast cells were seeded on to glass cover slips. After 72 hours of treatment with aqueous extract, the media was aspirated from the cell, fixed and stained with diluted Hoechst 33258 and photographed in fluorescent microscope (Leica – DMIL) at 340- 380 nm.
Wounding assays (Analysis of Cell Migration)
Fibroblasts were grown to confluence in 24 -well plates and wounded with a P200 pipette tip. After washing, plates were treated with different concentration of extracts. At an interval of 24 hours, the monolayer were photographed and wound width was measured using Qwin software.
Ammonium sulphate precipitation
For purification 10 g of aqueous powder was homogenized in 50 ml of 150 ml 50 mM potassium phosphate buffer pH.7 , filtered, centrifuged and fractionated by (NH4)2SO4 precipitation at 20,40,60,80 and 100% saturation. The precipitate was centrifuged, dialyzed and lyophilized to get the partially characterized protein.
Molecular weight determination of the active fraction
PAGE was carried out in a Genei mini model slab gel apparatus following the method of (Laemmli 1970) The protein content was estimated by the method of (Bradford 1976) The protein bands were located using coomassie brilliant blue stain.
Total-RNA extraction from endothelial cultures
RNA was isolated using trizole (Invitrogen 16096-020) reagent following the instruction manual of the kit. The total RNA was pelleted at 15,000 x g for 15 min at 4°C. The pellet was washed with 70% ethanol and resuspended in 30 µl of sterile DEPC-treated water. The sample was stored at -80°C until use. The RNA was quantified using nanodrop spectrophotometer (Nanodrop 8000).
Reverse transcription polymerase chain reaction (RT-PCR).
RT-PCRs were conducted using a Eppendorf PCR system (Eppendorf Canada) as per the instructions on the kit. For two-step RT-PCR, each reaction mixture (10 µl) contained 2 µl of first-strand buffer (fermentas), 0.3 mM deoxynucleoside triphosphates (dNTP), 5 ng of the random hexamer (fermentas) µl-1, 20 U of RNaseOUT, 10 mM dithiothreitol, 100 U of SuperScript II RNase H reverse transcriptase (fermentas), and 3 to 5 µl of RNA. The reaction mixture was incubated first at 42°C for 50 min and then at 70°C for 15 min. PCR assay was performed for 45 cycles with conditions, 95°C for 30 s, 52°C for 1 min, and 72°C for 45 s.
Statistical Analysis
All the quantitative data were analyzed by Student’s t –test and significance was assumed for P-values lower than 0.05.
Results
Effect of AEHA on fibroblast and endothelial cell proliferation
Effect of aqueous plant extract in fibroblast and endothelial proliferation was assayed by growing L929 monolayer in different concentration of the aqueous extract of Hemigraphis alternata (AEHA). The aqueous extract after filtration revealed the presence of 0.3mg/ml protein and 0.07mg/ml carbohydrate. Human fibroblast cell lines showed a dose dependent stimulation of growth by the AEHA in a serum free DMEM HG and serum containing DMEM HG. The untreated cells grown in DMEM HG with serum and with out serum were used as control experiment. The proliferative growth of cells was assayed by MTT method. The proliferative growth of cells in a range of concentration of AEHA with and without serum is shown in figure 1A and B. Enhancement in cell growth was observed from day 3 onwards on addition of the specific concentrations of AEHA between 0.15 mg/ml and 5.5 mg/ml. The optimal proliferation was observed for a concentration greater than 2.5 mg/ml irrespective to the presence or absence of serum. AEHA application with serum and with out serum revealed an increased proliferation compared to the control. To visualize the increase in cell number, the intact DNA was stained with Hoechst dye. The figure 1C demonstrates the qualitative increase in cell number as evident from the increased fluorescence of Hoechst dye in AEHA treated cells.
Endothelial cells isolated from human umbilical veins were identified with their cobblestone morphology and von willerbrand factor fluorescent antibody. The endothelial cells were also treated with the optimum concentration of AEHA as observed from fibroblast proliferation studies. Endothelial cells seeded at a density of 1500 cells per well in a 96 well plate and treated with different concentration of AEHA. AEHA at a concentration of 2.5mg/ml significantly increased cell proliferation by 50%. The concentration of 2.5mg/ml was found to be optimum concentration. The experiment was also done in serum free media, but endothelial cells do not survive long in the absence of serum. Media containing 20ng/ml VEGF was used as the positive control and media with out AEHA or VEGF was used as the negative control. A significant change in growth was observed from day 3 onwards in the cells treated with AEHA compared to negative control (Fig 1D). The cells grown in AEHA depict a slightly elongated morphology.
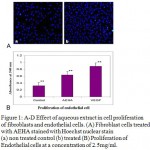 |
Figure 1: A-D Effect of aqueous extract in cell proliferation of fibroblasts and endothelial cells. (A) Fibroblast cells treated with AEHA stained with Hoechst nuclear stain (a) non treated control (b) treated (B) Proliferation of endothelial cells at a concentration of 2.5mg/ml. The results are shown as the mean ± standard deviation of three experiments *P<0.01compared with the control. |
Effect of protein from Hemigraphis alternata on cell proliferation
The protein from AEHA were fractionated and proliferative effect of each protein fraction was assayed, to study the involvement of proteins present in AEHA on cell proliferation. The total protein fraction showed a proliferative effect Among the protein fractions, 0-20%, 20-40%, 40-60%, 60- 80% and 80 -100% assayed, only 60% fraction revealed a proliferative effect at a concentration of 20mg/ml. To pin point the effect of this 60% protein fraction, only 60% precipitate was removed from AEHA by salting out. The cells grown in AEHA devoid of the 60% protein showed less proliferation (Fig 2A). Figure 2B demonstrates the comparison of proliferation of AEHA, 60% protein and AEHA devoid of protein. Though the protein fractions showed a proliferative effect, the rate of proliferation was insignificant as compared to the aqueous extract. The protein fractions had no effect on endothelial cells.
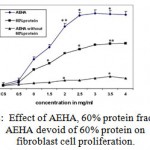 |
Figure 2: Effect of AEHA, 60% protein fraction and AEHA devoid of 60% protein on fibroblast cell proliferation. The results are shown as the mean ± standard deviation of three experiments *P<0.01compared with the control. |
Effect of AEHA on Cell migration
Stimulation of cell migration by AEHA and the protein fraction was performed by scratch wound assay using fibroblast L929 cell lines. Fibroblast cultures were maintained still confluence. Confluent cultures were wounded with P 200 pipette tips. The initial wound width was measured as 526 mm. Migration was measured as the reduction in wound width over a period of time 24, 48 and 72 hrs (Fig 3A & B). As shown in the figure, AEHA stimulated the migration of fibroblasts to about 60% compared to the control. The same experiment was repeated with protein fractions. The protein fractions showed only little effect in wound healing in comparison to that of the aqueous extract.
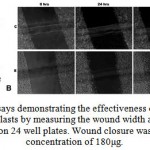 |
Figure 3: Wounding assays demonstrating the effectiveness of aqueous extracts. (A) Migratory rate of fibroblasts by measuring the wound width as a function of time for L929 cells seeded on 24 well plates. |
Wound closure was 97% for AEHA at concentration of 180μg.The control with out serum measured a wound closure to 4%. Data are expressed as the mean value of three experiments. Asterisks indicates the significant difference from control values.*, 0.01%, **, 0.01%. (B) Time lapse images at 24, 48 and 72 hours of control, and AEHA displayed an enhanced migration compared to the control. The photographs are taken with a 10x phase contrast objective.
Semi quantitative RT-PCR expression of angiogenic markers.
Angiogenesis plays a significant role during wound healing. Since endothelial cells showed significant increase in proliferation in the presence of AEHA and the plant is known for its wound healing properties, the expression of angiogenic markers on endothelial cells in the presence of AEHA were quantified by RT-PCR analysis. The primers were developed using primer 3 software and synthesized from eurogentec, Belgium. Table 1. The markers amplified are KDR, FLT 4, CD31 and VE Cadherin. The difference in amplification pattern is revealed in agarose gel (fig.4).
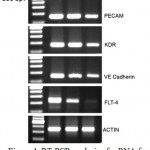 |
Figure 4: RT-PCR analysis of mRNA for endothelial cells. Rows demonstrated the expression of PECAM, KDR, VE cadherin, and FLT-4 gene expression compared to actin the internal control. Lane 1 –DNA ladder, Lane 2.- VEGF treated cells, Lane 3 – AEHA treated cells (2.5 mg/ml), Lane – 4. Negative control without any treatment. |
Discussion
Wounds in the tissues undergo a complex and ordered series of events like infiltration of inflammatory immune cells to destroy necrotic tissues, increased vascularization by angiogenic factors, increased cell migration, enhanced cell proliferation and extra cellular matrix deposition to repair tissue. Irrespective of their different origins, fibroblast also play an important role in wound healing through migration, proliferation, contraction and collagen production and through association of different protein matrix. In this study we have shown the invitro effect of Hemigraphis alternata in proliferation and migration of fibroblast cells. Fibroblast cell cultures have been proposed as a method for testing wound healing activity invitro (Graham., et al 1984). Despite a history of Hemigraphis alternata in oriental medicine as a wound healing agent, there is little experimental evidence supporting this speculation. MTT assay revealed moderately enhanced proliferation of fibroblast and endothelial cells when treated with AEHA. The extracts of different plant extracts such as Uncaria rhynchophylla (Choi., et al 2005), Emblica officianalis and Hedychyum spicatum (Dev 1999) are reported to have an inducing effect on the growth of endothelial cells as well as fibroblast cells (Steenkamp et al., 2004). The alcoholic extract of Tinosporia cordifolia is also reported to have an effect in the proliferation and myeloid differentiation of bone marrow precursor cells (Singh et al 2006). To determine the growth effect of AEHA more precisely and to significantly check the role of serum in AEHA action, proliferation experiments were carried out with AEHA in serum supplemented and serum free medium. In both cases, the concentration of AEHA showed an increased performance from a concentration of 2.5mg/ml or greater in serum free medium and serum containing medium. Hence it is inferred that both in the presence and absence of serum, the different molecules of AEHA are expected to synergistically aid the action of cell proliferation. The proliferative action of the extract can be correlated to the absorbance (OD) value obtained control-0.421, without serum-0.749 and with serum-1.27nm. Only 60% protein precipitate from AEHA showed a proliferation as exhibited from figure 2A & B and confirmed to be a protein having molecular weight of 21.8 kD. The proliferation of AEHA is a synergistic effect of the 60% protein and other water soluble molecules like amino acids, flavenoids and other alkaloids. It is tempting to speculate that the influence of AEHA on optimum cell growth points to the direct involvement of the AEHA in cell metabolism. The enhancement in cell number in the presence of AEHA can be visualized by the increase in the nuclei stained with Hoechst dye. The 21.8 kD protein falls in the molecular weight range of other growth factors VEGF – 38.2 kDa, EGF- 6kDa etc. So we hypothesize that this protein may be mimicking some growth factors since the presence of protein is also found to be necessary for the action of AEHA in the case of fibroblast cells. However while the aqueous extract (AEHA) showed a significant increase in endothelial cell proliferation, the protein fractions showed no proliferation effect on endothelial cells. Thus its quiet clear that the aqueous crude extract of the plant also contain some minor fraction of constituents that synergistically work with the protein fraction of the extract in inducing wound healing since AEHA is having significant effect on both fibroblasts and endothelial cells.
Scratch wound cell migration assay revealed the positive effect of the aqueous extract in cell migration (60% migration). We found that wound healing was observable between 3 and 24 hrs after wounding with a pipette tip, significant cell migration could be seen at 3 hrs with lamella and protrusions at the wound margin. The cell migrations in the presence of different fractions of proteins were also assayed. The protein fractions revealed a migration below 15%. Since AEHA showed cell migration and protein fraction did not, it is expected that some other water soluble molecules could also be promoting cell migration.
Wound angiogenesis is an important part of the proliferative phase of healing; with the appearance of the prominent blood vessels of the initial connective tissue formed in the wound space. The increased expression of FLT 4, KDR, CD31 and VE cadherin is observed in endothelial cells when treated with AEHA. The growth factor receptors expressed on endothelial cells are of special interest because of their potential to program endothelial cell growth and differentiation during development and neovascularization in various pathological states, especially angiogenesis during wound healing. The expression of the receptors FLT 4 and KDR regulates the role of VEGF necessary for the endothelial monolayer recovery. So the expression of KDR and FLT 4 in the presence of AEHA can be the correlated to VEGF expression and recovery of cells after wounding. PECAM over expression is also noticed in endothelial cells treated with AEHA. PECAM is involved in endothelial cell migration and angiogenesis. Role of PECAM in endothelial migration and angiogenesis via different pathways are well referred (DeLisser., et al 1997, Lu., et al 1996, Paik., et al 2001). VE cadherin is also found to be over expressed in the presence of AEHA. VE Cadherin is having a typical functional role in cell adhesion. Different functional role of VE cadherin in the maintenance of endhothelial cell is known (Breviario.,et al 1995, Carmeliet., et al 1999). VE-cadherin is an endothelial-specific cadherin that plays a central role in vascular barrier function and angiogenesis. Adhesive intercellular junctions mediate contact between adjacent cells and provide positional cues to cells during development, wound healing, and other processes that require dynamic cell-cell contact (Marrs and Nelson 1997, Takeichi., 1995 , Tepass., et al 2000 , Yost et al 1996). Cell adhesion is also important in angiogenesis. The adhesion and migration processes are maintained by the joint action of PECAM and VE Cadherin. Hence the over expression of PECAM and VE Cadherin when treated with AEHA correlates to the role of the extract on endothelial cell migration and adhesion for wound healing.
The wound healing property known about Hemigraphis alternata can be due to this proliferation and migration of endothelial and fibroblast cells. Since AEHA is inducing proliferation of fibroblast cells and endothelial cells, the promitotic effect of the extract could be one of the reasons for wound healing activity of this plant. As the angiogenic markers are expressed in endothelial cells, the plant extract is also expected to promotes angiogenesis which is a significant part of wound healing. Thus the findings clearly evaluate the effectiveness of the plant extract as a potential wound healing agent. The less effectiveness of protein fraction compared to AEHA suggests different molecules in the plant are synergistically acting on wound healing. These data present the first in vitro evidence that Hemigraphis alternata significantly induces wound healing. In addition it is expected that this study will allow better understanding of the herbal drug promoted migration and proliferation in wound healing. Quantitative characterization of AEHA for different active components in detail and effect of these components in mammalian cells are the future research objectives regarding this plant.
Acknowledgements
The authors are grateful to Director of SCTIMST; Head of BMT wing for providing infrastructural facilities, BT Edwin acknowledges the fellowship from DBT and KSCSTE.
Reference
- Kumara,B., Vijayakumar ,M., Govindarajan, R., Pushpangadan, P., 2007. Review Ethnopharmacological approaches to wound healing—Exploring medicinal plants of India. Journal of Ethnopharmacology 114,103–113.
- Puratchikody, A., Nithya Devi., Nagalakshmi., 2006. Wound healing activity of Cyperus rotundus Linn. Indian Journal of Pharmacology 68 (1), 97-101.
- WHO Traditional medicine strategy 2002-2005, WHO Geneva.
- Sukh Dev., 1999. Ancient – Modern Concordance in Ayurvedic Plants: Some Examples Environmental Health Perspective 107 (10), 783-789.
- Subramoniam, A., Evans, D.A., Rajasekharan, S., Nair, G.S., 2001. Effect of Hemigraphis colorata (Blume) H.G. Hallier leaf on wound healing and inflammation in mice. Indian Journal of Pharamcology 33, 283–285.
- Lawrence, W. T., Diegelmann, R. F., 1994. Growth factors in wound healing. Clinical Dermatology 12, 157-162.
- Kim, W. J., Gittes, G. K., Longaker, M. T., 1998. Signal transduction in wound pharmacology. Archives of Pharmacological Research 21, 487-492.
- Gillitzer, R., Goebeler, M., 2001.Chemokines in cutaneous wound healing. Journal of Leukocyte Biology 69, 513-519.
- Nilgun Ozturk., Seval Korkmaz., Yusuf Ozturk., 2007. Wound healing activity of St.John’s Wort (Hypricum perforatum L) on chicken embryonic fibroblasts. Journal of Ethnopharmacology 111(1), 33-39.
- Layne, E.,1957. Spectrophotometric and turbidimetric methods for measuring proteins. Methods in enzymology (Vol. 3, pp. 450). New York: Academic press, Ind
- Morris, D.L., 1948. Quantitative Determination of Carbohydrates with Dreywood’s Anthrone Reagent. Science 107, (2775) 254 – 255.
- Jaffe, E.A., Nachman, R.L., Becker., C.G.Minick, C.R., Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. Journal of Clinical. Investigation 52, 2745–2756
- Mosmann T., 1983.Rapid colorimetric assay for cell growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65, 55-63.
- Niks, M., Otto, M., 1990. Towards an optimized MTT assay. Journal of Immlaunological Methods 130 (1),149-151.
- Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the Head of Bacteriophage T4. Nature 227, 680-685.
- Bradford, M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye-binding. Analytical Biochemistry 72, 248-254.
- Graham, M.F., Diegelmann, R.F., Cohen, I.K., 1984. An in vitro model of fibroplasia: simultaneous quantification of fibroblast proliferation, migration, and collagen synthesis. Proceedings of the Society for Experimental Biology and Medicine 176, 302-308.
- Choi,S.W., Son,B.W., Son,Y.S., Park, Y.I., Lee,S.K., Chung, M.H., 2001.The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. British Journal of Dermatology 145 (4), 535-545.
- Steenkamp,V., Mathivha, E., Gouws, M, C., Van Rensburg, C E., 2004. Studies on antibacterial,antioxidant and fibroblast growth stimulation of wound healing remedies from south Africa. Journal of Ethnopharmacology 95, 353- 357.
- Sukh Mahendra Singh., Nisha Singh., Pratima Shrivastava., 2006. Effect of alcoholic extract of Ayurvedic herb Tinospora cordifolia on the proliferation and myeloid differentiation of bone marrow precursor cells in a tumor-bearing host Fitoterapia 77(1), 1-11.
- DeLisser, H. M., Christofidou-Solomidou, M., Strieter, R. M., Burdick, M. D., Robinson, C. S., Wexler, R. S., Kerr, J. S., Garlanda, C., Merwin, J. R., Madri, J. A., 1997. Involvement of endothelial PECAM-1/CD31 in angiogenesis. American Journal of Pathology 151,671-677.
- Lu, T. T., Yan, L. G., Madri, J. A., 1996. Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule 1. Proceedings National Academy of Sciences USA 93, 11808-11813.
- Paik, J. H., Chae, S., Lee, M. J., Thangada, S., Hla, T.,2001. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. Journal of Biological Chemistry 276,11830-11837.
- Breviario, F., Caveda, L., Corada M., Martin-Padura, I., Navarro, P., Golay, J., Introna, M., Gulino, D., Lampugnani, M.G., and Dejana,E., 1995. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arteriosclerosis Thrombosis and Vascular Biology 15, 1229-1239.
- Carmeliet, P., Lampugnani, M.G., Moons, L., Breviario, F., Compernolle, V., Bono, F., Balconi, G., Spagnuolo, R., Oostuyse, B., Dewerchin, M., Zanetti, A., Angellilo, A., Mattot, V., Nuyens, D., Lutgens, E., Clotman, F., de Ruiter,M.C., Gittenberger-de Groot, A., Poelmann, R., Lupu, F., Herbert, J.M., Collen, D., Dejana, E., 1999.Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98,147-157.
- Marrs, J.A., Nelson,W.J., 1996.Cadherin cell adhesion molecules in differentiation and embryogenesis. International Review of Cytology 165, 159-205.
- Takeichi, M., 1995.Morphogenic roles of classic cadherins. Current Opinion in Cell Biology 7, 619-627.
- Tepass, U., Truong, K., Godt, D., Ikura, M., Peifer, M., 2000. Cadherins in embryonic and neural morphogenesis. Nature Reviews Molecular Cell Biology 1, 91-100.
- Yost, C., Torres, M., Miller, J,R., Huang, E., Kimelman, D., Moon, R,T., 1996. The axis-inducing activity, stability, and subcellular distribution of -catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes & Development 10, 1443-1454

This work is licensed under a Creative Commons Attribution 4.0 International License.





