Manuscript accepted on : July 09, 2011
Published online on: 28-06-2011
Lipid Transport in Plasmodium: Role as Possible Target for Novel Chemotherapy Against Malaria
N. N. Nwobodo1*, P. O. Okonkwo2 and S. A. Igwe1
1Department of Pharmacology and Therapeutics, College of Medicine, Enugu State University of Science and Technology, Enugu Nigeria.
2Department of Pharmacology and Therapeutics, College of Medicine, University of Nigeria, Enugu Nigeria.
ABSTRACT: Lipid trafficking pathways in malaria-infected erythrocytes are complex because the malaria parasite is separated from the serum by the erythrocyte and parasitophorous vacuolar membrane (PVM). The PVM lipids in malaria-infected erythrocytes are derived from host cells. Lipid rafts which are cholesterol and sphingolipid enriched membrane domains appear to be involved in malaria infection. Thus, perturbation of lipid raft specific lipids in the host erythrocyte membrane can influence the cell’s ability to be infected. This paper attempts to discuss novel approaches in the treatment of malaria infection by targeting and manipulating host cell lipids based on recent discoveries on the role of lipid rafts in malaria pathogenesis.
KEYWORDS: Lipid transport; malaria; novel chemotherapy; plasmodium; possible target
Download this article as:| Copy the following to cite this article: Nwobodo N. N, Okonkwo P. O, Igwe S. A. Lipid Transport in Plasmodium: Role as Possible Target for Novel Chemotherapy Against Malaria. Biosci Biotech Res Asia 2011;8(1) |
| Copy the following to cite this URL: Nwobodo N. N, Okonkwo P. O, Igwe S. A. Lipid Transport in Plasmodium: Role as Possible Target for Novel Chemotherapy Against Malaria. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9300 |
Introduction
The mature human er ythrocyte is a terminally differentiated simple cell that is devoid of intracellular organelles and does not show endocytic or phagocytic activity. It is readily invaded by malaria parasites which involute the red cell to generate a host-derived parasitophorous vacuolar membrane (PVM).
It is now evident that in Plasmodium- infected erythrocytes lipid rafts play a role in endovacuolation and macromolecular transport1. Host cholesterol is also detected in the PVM and depletion of erythrocyte cholesterol which disrupts DRM (detergent resistant membrane) complex, but has no effect on membrane deformation blocks infection. It is interesting to note that erythrocyte raft lipids recruited to the invasion junction by
mechanical stimulation may be remodeled by the malaria parasite to establish blood-stage infection. Research findings suggest that TVM is a transport network that allows lipids efficient access to the parasite and could be used to deliver anti-malarial drugs directly into the parasite2.
It has been shown that drug-induced endovesicles show all cholesterol characteristics of rafts and also contain phosphatidylserine (PS) and phosphatidylinositol-4,5-bisphosphate (PIP ). A study employed lipid mass spectrometry which reveals that phosphatidylethanolamine and phosphatidylglycerol are depleted in endovesicles while phosphoinositides are highly enriched, suggesting raft-based endovesiculation can be achieved by simple (non-receptor mediated) mechanical pertubation of the erythrocyte plasma membrane and results in sorting of inner leaflet phospholipids3. presence of PIP on budding membranes can also Notably, PIP is a major phosphoinositide inhibit membrane fission processes required for endosomes to pinch off from membranes, and thus (PI) in erythrocyte membranes and influences PIP removal may be needed to create a larger junctional complex stability. The same study vacuole by the malaria parasite which is critical for evaluated the presence of raft lipids PIP and PS in establishing erythrocyte infection. the malarial vacuole by tagging them with specific fluorescently labeled protein reagents in re-sealed erythrocyte ghosts whose signaling properties and infection by malaria parasites are comparable to nor mal intact er ythrocytes4 . It is found,
The human malaria parasite, during intra- erythrocytic development, actively internalizes phospholipid from its erythrocyte membrane and the extracellular medium. The import of exogenous unexpectedly, that PIP is excluded from the lipids is not due to endocytosis but to energy- vacuole, while PS is detected in newly formed PVM (parasitophorous vacuole membrane), thus providing the first evidence for er ythrocyte phospholipid remodeling on the cytoplasmic surface of the malarial vacuole. Thus, it is shown that PIP is differently remodeled in distinct raft-based endovesicles/vacuoles induced in the erythrocyte. The data strongly suggest that major raft lipids may be enriched in endovesicles upon simple mechanical pertubation of the bilayer. However, in pathogen induced endovacuolation such as in malarial infection, specific raft lipids are actively excluded from the vacuole, suggesting a new model for erythrocyte raft movement in invasion. A previous study showed that exogenous, chemically labeled PE lipids introduced into erythrocytes are excluded
dependent, trans-bilayer movement of phospholipids induced by the parasite in the erythrocyte surface7. Novel tubular membrane that appears to emerge from the vacuole of the parasite and extends into the erythrocyte cytoplasm are labeled by exogenously added fluorescent lipids. It is shown that both biochemical and microscopic studies indicate that all lipid analogs internalized into intra-erythrocytic compartments and/or the parasite are not exported back to the host cell surface. A study described the existence of two distinct pathways for transport of macromolecules (lipids) in the external medium or host cell cytosol to the intracellular parasite8. It addressed the purity of the fluorescent macromolecules used to define the parasitophorous duct pathway; and provided from PVM5. A study reported that PIP provides the ultrastructural evidence for its presence. The first report of an intrinsic erythrocyte raft lipid excluded from the PVM, made possible by a new ghost-loading approach that allows experimental fluorescent tracers used to characterize transport remain intact during their incubation with infected erythrocytes. Transmission electron microscopy in access to cytoplasmic PIP and PS4. Since PIP is that study revealed areas of membrane continuity cytoplasmically oriented, sorting/remodeling signals must be transduced to the cytoplasmic leaflet of the erythrocyte bilayer.
However, whether exclusion is linked to signaling via an erythrocyte ghost known to be harnessed by malaria parasite or to additional lipid signaling pathways needs to be investigated further. However, it is noted that PIP is critical for endocytic processes in many cell types. Phosphatidylinositols (PIs) interact with the actin cytoskeleton, ion channels and act on effectors following head group
between the er ythrocyte membrane and the parasitophorous vacuolar membrane which may constitute the “metabolic window” hypothesized to occur at contact sites or region of close apposition between the erythrocyte membrane and PVM9. It has been reported that in the erythrocyte cytosol a var iety of tubular and vesicular membrane structures, are thought to extend out from the PVM and variously referred to as “tubovesicular membrane” or tubovesicular network10.
A study described newly elaborated carbon hydrolysis6. Consequently, loss of PIP from the dense tubular and sheet-like structures that appear vacuole by phosphatases, kinases or lipases of either host or parasite origin may enable active clearance of erythrocyte skeleton to propagate the early vacuole and ensure infection. The continued to surround the parasite and extend into the red cell cytosol11. It is hypothesized that these structures are components of the tubovesicular network (TVN), a network that is thought to play a prominent role in lipid transpor t. Another study described that extracellular solutes such as the lucifer yellow enter the TVM and are delivered to the parasite. Blocking the assembly of the network, blocks the delivery of exogenous lucifer yellow. Findings suggest that the TVM is part of the secretory pathway involved in lipid transport across the Plasmodium infected erythrocyte12.
Sphingolipid synthesis in the infected red cell has been localized to the TVN which may be analogous to the cis-Golgi13. Parasite Golgi activities for the synthesis and accumulation of sphingomyelin are detected in the intra-erythrocytic tubules, indicating a novel export of classic secretory functions to their lumen, which could be central to both tubular development and lipid sorting activities in these organelles. Some data strongly suggest that transport in infected red cell can proceed via a classical Golgi secretory pathway14 or a functional but reduced Golgi15. Interestingly, other researchers hypothesize that a mor phologically and biochemically distinct “classical” Golgi complex may not exist16. A study has shown that macromolecules (lipids) do not cross the er ythrocyte or parasitophorous vacuolar membranes, but rather gain direct access to the aqueous space surrounding the parasite through a parasitophorous duct17. However, another study yielded results indicating the presence of parasite induced mechanisms of lipid transport in infected erythrocyte membranes that modify host membrane properties and may have impor tant implications on phospholipid asymmetry in these membranes. The study reveals that the internalization of NBD-PC[(1- palmitoyl-2-[6-[7-nitro-2-1, 3-benzoxadiazole-4-yl)
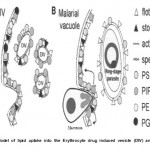 |
Figure 1: Model of lipid uptake into the Erythrocyte drug induced vesicle (DIV) and Malarial vacude.
|
(Modified and Adapted from Murphy S.C, Fernandez-Pol S., Chung P.H., Prasanna-Murthy S.N., Milne S.B., Salomao M., Brown H.A., Lomasney J.W., Mohandas N., Haldar K. Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malaria parasite Plasmodium falciparum. Blood. 110(6): 2138, 2007) amino] caproyl)] phosphatidylcholine is not because of endocytosis but rapid transbilayer flip-flop at the infected erythrocyte membranes, followed by monomer diffusion to the parasite18.
Nevertheless, much remains to be learned about the nature of the pathways for lipid trafficking between the parasite and external milieu. The physical nature of routes for transport of nutrients and lipid macromolecule has been debated. There may be a transient or stable parasitophorous duct of 50-70nm diameter, which provides direct access between the intra-erythrocytic parasite and the external milieu. Its existence was first suggested by the results of a study that used small highly fluorescent latex spheres to investigate transport of macromolecules18. The above findings have been contradicted by other studies19,20. However, it has been suggested that lipid distribution in the erythrocyte membrane may be significantly more ordered than previously expected, such that simple mechanical per tubation may enable highly specialized domains to energetically separate from the rest of the erythrocyte membrane.
New permeability pathways (NPP) in the intra-erythrocytic stage to supply nutrients (lipids) to the parasite across the red cell membrane are under investigations as drug targets. The NPPs are visualized to function in enhancing supply of nutrients or to deliver drugs as well as to efflux excess metabolites21. However, lack of correlation between the inhibitory activity on NPP and parasite growth, and non-selectivity due to inhibition of the red blood cell pathway component have been reported. NPPs can be used for selective targeting of anti-malarials2.
Conclusion
Conclusively. lipid transport systems in the malaria parasite facilitate access of the much needed lipids for building up its cell membrane. Consequently, they could serve as potential targets for novel anti-malarial chemotherapy.
Refrences
- Haldar , Samuel B.U., Mohandas N., Harr ison T. and Hiller N.L. Transpor t mechanisms in plasmodium infected erythrocytes: lipid rafts and a tubovesicular network. Int. J. Parasitol. 31(12): 1393-1401 (2001).
- Lauer S.A, Rathod P.K., Ghori N. and Haldar A membrane network for nutrient import in red cells infected with the malaria parasite. Science. 276(5315): 1122-1125 (1997).
- Murphy S.C., Fernandez-Pol S., Chung P.H., Murthy S.N.P., Milne S.B., Salomao , Brown H.A., Lomasney J.W., Mohandas N. and Haldar K. Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malar ia parasite Plasmodium falciparum. Blood. 110: 2132-2139 (2007).
- Murphy S.C., Harrison T., Hamm E., Lomasney J.W., Mohandas N. and HaldarErythrocyte G protein as a novel target for malaria chemotherapy. PloS. Med. 3: e528 (2006).
- Dluzewski R., Mitchell. G.H., Fryer P.R., Griffiths S., Wilson R.J. and Gratzer W.B. Origins of the parasitophorous vacuole membrane of the malar ia parasite Plasmodium falciparum, in human red blood cells. J. Cell Sci. 102: 527-552 (1992).
- Czech P. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu. Rev. Physiol. 65: 791-815 (2003).
- Haldar K. Lipid transport in Infect. Agents Dis. 1(15): 254-262 (1992).
- Goodyer D., Pouvelle B., Schneider T.G., Trelka D.P. and Taraschi T.F. Characterization of macromolecular transport pathways in malaria infected erythrocytes. Mol. Biochem. Parasitol. 87(1): 13-28 (1997).
- Elford B.C, Cowen M. and Ferguson D. Parasite regulated membrane transport process and metabolic control in malaria- infected erythrocytes. Biochemical Journal. 308: 361-374 (1995).
- Kirk K. Membrane transpor t in malaria- infected erythrocytes. J. Exp. Med. 1(2): 495- 537 (2001).
- Magowan C., Brown J.T., Liang J., Heck J., Coppel L., Mohandas N. and Meyer-Ilse W. Intracellular structures of normal and aberrant Plasmodium falciparum malaria parasite imaged by soft x-ray microscopy. Proct. Natl. Acad. Sci. USA. 94: 6222-6227 (1997).
- Bracho C., Dunia I, D e R., Benedetti E.L. and Perez H.A. Traffic pathways of Plasmodium vivax antigens during intra- erythrocytic parasite development. Parasitol. Res. 88(3): 253-258 (2002).
- Elmendorf G. and Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO.J. 12: 4763-4773 (1993).
- Hinterberg , Scherf A., Gysin J., Toyoshima T., Aikawa M., Mazie J.C., da Silva L.P. and Mattei D. Plasmodium Falciparum: the Pf332 antigen is secreted from the parasite by a brefeldin a-dependent pathway and is translated to the erythrocyte membrane via the mauver’s clefts. Exp. Parasitol. 79: 279- 291(1994).
- Ward , Tilney L.G. and Langsley G. Rab GTPases and the usual secretory pathway of Plasmodium. Today. 13: 57-62 (1997).
- Banting , Banting J. and Lingelbach H.K. A minimalist view of the secretory pathway in Plasmodium falciparum. Trends in Cell Biology. 5: 340-343 (1995).
- Pouvelle B., Spiegel , Hsiao L., Howard R.J., Morris R.L., Thomas A.P. and Taraschi T.F. Direct access to serum macromolecules by intra-er ythrocytic malaria parasites. Nature. 353(6339): 73-75 (1991).
- Cross A., Amorim de A.F. and Haldar K. Transpor t of fluorescent phospholipid analogues from the erythrocyte membrane to the parasite in Plasmodium falciparum– infected cells. The Journal of Cell Biology. 108: 2183-2192 (1989).
- Fujioka H. and Aikawa M. Morphological changes of clefts in Plasmodium-infected erythrocytes under adverse conditions. Exp. Parasitol. 76: 302-307 (1993).
- Hibbs , Stenzel D.J. and Saul A. Macromolecular transport in malaria-does the duct exist? Eur. J. Cell Biol. 72: 182-188 (1997).
- Staines M., Ellory J.C. and Chibale K. The new permeability pathways: targets and selective roles for the development of new anti-malarial agents. Combinatorial Chem. High Throughout Screening. 8: 81-88 (2005

This work is licensed under a Creative Commons Attribution 4.0 International License.





