Manuscript accepted on : January 06, 2011
Published online on: 28-06-2011
Jirapha Keeddee, Numpol Rattanaworasin and Montree Tungjai*
Center of Excellence for Molecular Imaging, Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai - 502 00 Thailand.
Corresponding Author E-mail: montree@chiangmai.ac.th
ABSTRACT: The natural products have been reported to exhibit anticancer and antioxidant activities. The aim of this study was to determine the effect of 5,4'-dihydroxy-3,6,7,8-tetramethoxyflavone (WP279), 5,5'-dihydroxy-6,7,3',4'-tetramethoxyflavone (WP280) and 5,3'-dihydroxy-3,6,7,8,4'-pentamethoxyflavone(WP283) on P-glycoprotein-mediated transport and intracellular reactive oxygen species in K562 and K562/adr (P-glycoprotein overexpression) cancer cells. The ability to inhibit of P-glycoprotein-mediated transport of pirarubicin out of the cells was determined using non-invasive functional spectrofluorometric techniuqe. The ratio of kai / ka0 value was used to indicate the ability of molecule to inhibit the P-glycoprotein active efflux of a drug that if kai / ka0 = 1 or 0, these mean that there was not inhibition and was completely blocked P-glycoprotein function respectively. The three methoxyflavones could partially inhibit function of P-glycoprotein active efflux of a drug by kai / ka0 values equal to 0.3-0.1 approximately. For the effect of three methoxyflavones on intracellular reactive oxygen species in both cancer cells was also determined by using 2', 7'-dichlorofluorescein diacetate as a fluorescent probe. The involved parameters in dichlorofluorescein formation, k2ROSi could be quantitatively determined by fitting mathematically equation to the experimental spectrofluorometric data. WP279 WP280 and WP283 could deplete k2ROSi in K562 cells by about 53 %, 55% 38% and K562/adr cells by 54%, 67%, and 58%, respectively. The three methoxyflavones decreased in intracellular reactive oxygen species in both cancer cells as a concentration dependent mannerThe natural products have been reported to exhibit anticancer and antioxidant activities. The aim of this study was to determine the effect of 5,4'-dihydroxy-3,6,7,8-tetramethoxyflavone (WP279), 5,5'-dihydroxy-6,7,3',4'-tetramethoxyflavone (WP280) and 5,3'-dihydroxy-3,6,7,8,4'-pentamethoxyflavone(WP283) on P-glycoprotein-mediated transport and intracellular reactive oxygen species in K562 and K562/adr (P-glycoprotein overexpression) cancer cells. The ability to inhibit of P-glycoprotein-mediated transport of pirarubicin out of the cells was determined using non-invasive functional spectrofluorometric techniuqe. The ratio of kai / ka0 value was used to indicate the ability of molecule to inhibit the P-glycoprotein active efflux of a drug that if kai / ka0 = 1 or 0, these mean that there was not inhibition and was completely blocked P-glycoprotein function respectively. The three methoxyflavones could partially inhibit function of P-glycoprotein active efflux of a drug by kai / ka0 values equal to 0.3-0.1 approximately. For the effect of three methoxyflavones on intracellular reactive oxygen species in both cancer cells was also determined by using 2', 7'-dichlorofluorescein diacetate as a fluorescent probe. The involved parameters in dichlorofluorescein formation, k2ROSi could be quantitatively determined by fitting mathematically equation to the experimental spectrofluorometric data. WP279 WP280 and WP283 could deplete k2ROSi in K562 cells by about 53 %, 55% 38% and K562/adr cells by 54%, 67%, and 58%, respectively. The three methoxyflavones decreased in intracellular reactive oxygen species in both cancer cells as a concentration dependent manner
KEYWORDS: Methoxyflavone, P-glycoprotein (P-gp); Intracellular reactive oxygen species (ROSi)
Download this article as:| Copy the following to cite this article: Keeddee. J, Rattanaworasin. N, Tungjai. M. The Effect of Methoxyflavone on P-Glycoprotein- Mediated Transport and Intracellular Reactive Oxygen Species in Cancer Cells. Biosci Biotechnol Res Asia 2011;8(1) |
| Copy the following to cite this URL: Keeddee. J, Rattanaworasin. N, Tungjai. M. The Effect of Methoxyflavone on P-Glycoprotein- Mediated Transport and Intracellular Reactive Oxygen Species in Cancer Cells. Biosci Biotechnol Res Asia 2011;8(1) . Available from: https://www.biotech-asia.org/?p=9161 |
Introduction
Multidrug resistant (MDR) is a major problem in the treatment of cancer. MDR is classically used to define a resistance phenotype where cells become resistant simultaneously to different drugs with no obvious structural resemblance and with different cellular targets (1). One widely study mechanism of these multidrug resistance transporters involves the ability of an ATP dependent 170 kDa plasma membrane protein, P-glycoprotein (P-gp), to act as an efficient efflux pump against anticancer drug molecules (2). Compounds such as cyclosporin A ,verapamil have been reported to reverse MDR, and increased accumulation of antitumor agents in MDR cells. However, these compounds are high toxicity and up to date no chemosensitisers can be used at clinical level (3). Nowadays, natural product is focusing for anticancer agent. Flavonoids such as quercetin, keampferol, apigenin, are found in several natural products (4). Many researchers have conducted in vitro and in vivo studies on the potential anticancer activity of flavonoids in several cancer cell types. (5,6,7,8). Choiprasert et al. study the effect of quercetin, quercitrin and rutin on MDR protein function in multidrug resistance cancer cell. The result is found that quercetin and quercitrin can inhibit P-glycoprotein function in K562/adr cell and MRP1 function in GLC4/adr cell (9). Kaempferol, quercetin, baicalein, myricetin, fisetin, and morin increase accumulation of daunorubicin, substrate of P-gp, in multidrug-resistant P-gp overexpressing KB-C2 cells (10). Flavonoids are not only effect on MDR protein but also on reactive oxygen species (ROS) too. Rithidech et al. study antioxidant property of apigenin in human lymphocyte. They find that a significant increase in the frequency of micronucleus in irradiated cells however; the frequency is decreased as the concentration of apigenin increased (11).
We have previously determined the speciation of the methoxyflavone including 5,4′-dihydroxy-3,6,7,8-tetramethoxyflavone, 5,5′-dihydroxy-6,7,3′,4′-tetramethoxy flavones and 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone in a physiological solution and anticancer property. The molecules exhibited the similarly anticancer activity in drug-resistant K562/adr cells which over expressed P-glycoprotein (P-gp) and GLC4/adr cells which over-expressed MRP1 protein when compared with their corresponding drug-sensitive cancer cell lines (12). It hinted us to hypothesize that theses methoxyflavones might be act as a MDR reversing agent. In this study, we determined the effect of three methoxyflavones on P-glycoprotein-mediated transport and also intracellular reactive oxygen species in cancer cells.
Materials and Methods
Drugs and chemicals
The 5,4′-dihydroxy-3,6,7,8-tetramethoxyflavone (WP279), 5,5′-dihydroxy-6,7,3′,4′-tetramethoxyflavone (WP280) and 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (WP283) (see chemical structure in figure 1) that isolated from Gardenia obtusifolia Roxb were kindly provided by Assoc. Prof. Dr. Wilart Poompimon, Department of Chemistry, Faculty of Science, Lampang Rajabhat University, Lampang, Thailand. 2′, 7’-dichlorofluororescin diacetate (DCHF-DA) was obtained from Invitrogen (CA, USA). Pirarubicin were from Sigma.
Cell lines and culture conditions
Adriamycin-sensitive erythromylogenous leukemic cells (K562), adriamycin-resistant erythromylogenous leukemic cells (K562/adr, overexpressed P-glycoprotein,(P-gp)) (13) were grown in RPMI-1640 medium supplemented with 1% penicillin-streptomycin and 10 % fetal calf serum (Gibco Biocult Ltd.) in a humidified incubator of 95% air, 5% CO2, 370C. For routine culture, a number of 105cells/mL grew exponentially to about 8-10 x 105cells/mL in 72 hours. For experiments, cells were initiated at 5 ´ 105 cells/mL and reached a number of about 8-10 ´ 105 cells/mL for 24 hours. These cells were in the exponential growth phase.
Determination the kinetic of the P-glycoprotein-mediated efflux pirarubicin
The kinetic of the P-glycoprotein-mediated efflux pirarubicin was measured using a non-invasive functional spectrofluorometric (Perkin Elmer) method which could be used to monitor a P-glycoprotein function in drug-resistant cancer cells as previously described by Mankhetkorn et al. (14). Briefly, Cells (2×106 cells) were incubated in 2 mL HEPES Na+-buffer, pH 7.25 in the absence of glucose and presence 10 mM NaN3 under continuous stirring at 37 0C in 1-cm quartz cuvette for 30 min. Pirarubicin (1 mM) was added to cell (CT), the initial fluorescence intensity was defined as F0. The fluorescence intensity of pirarubicin at 590 nm (excite at 480 nm) was recorded as a function of time. Fluorescence intensity was decreased during observation due to pirarubicin intercalated between the DNA base pairs. In condition intracellular pH (pHi) = extracellular pH (pHe), the intracellular free drug concentration (Ci) was equal to the free drug concentration in the extracellular medium (Ce) at the first steady state. At the steady state, a various concentration of molecule was added and followed by 5 mM glucose. After addition of 5 mM of glucose, the florescence intensity was increased. The slope of the tangent to the curve was dF/dt (tglu) and Va was the kinetic of release of pirarubicin, Va = dF/dt (tglu) x CT/F0, mM.s-1. ka was the P-glycoprotein-mediated efflux coefficient of drug, ka = Va / (n x CT-Cn) in s-1 where n was the number of cell. In the following, kai and ka0 were the P-glycoprotein-mediated efflux coefficient in the presence and in the absence of molecule, respectively. The ratio of kai / ka0 was used to indicate the ability of molecule to inhibit the P-glycoprotein active efflux of a drug. kai / ka0 = 1, this mean that there was not inhibition of active efflux, and = 0 , the P-glycoprotein active efflux was completely blocked.
Measurement of intracellular reactive oxygen species (ROSi)
The formation of ROSi measured using 2¢, 7¢-dichlorofluorescein diacetate (DCHF-DA) as a fluorescent probe was described by Loetchutinat et al. (15). Briefly, Cells (105 cell/mL) were incubated with molecule in 2 mL HEPES Na+-buffer, pH 7.25 at 37 0C under vigorous stirred for 5 minutes. Then, CoCl2 (20 mM) was added to quench extracellular dichlorofluorescein (DCF) fluorescence and following by 100 nM DCHF-DA. The DCF fluorescence intensity at 523 nm (excite at 502 nm) was recorded as a function of time.
DCHF-DA was hydrolyzed by intracellular esterase to non-fluorescent 2¢,7¢-dichlorofluorescein (DCHF). DCHF reacted with ROSi to form fluorescent DCF. The overall of reaction could be written as following:
DCHF-DA DCHF (1)
DCHF + ROSi DCF (2)
The model was mathematically expressed in equation:
[DCF] = [DCHF-DA]0 x (k2[ROSi](1-e-kt ) – ke(1-ek2[ROSi]t)) / (k2[ROSi] – ke (3)where ke = k1[E]0 was the pseudo-first-order rate constant for reaction (1) (s-1).
The parameters intervening in DCF formation, ke and k2[ROSi], could be quantitatively determined by fitting equation (3) to the experimental spectrofluorometric data.
Results and Discussions
Inhibition of P-gp-mediated efflux pirarubicin out of MDR cells in the presence of WP279, WP280 and WP283
The ability of P-gp to pump pirarubicin out of K562/adr cells was determined in the presence and absence of WP279, WP280 and WP283. A typical experiment was shown in figure 2. The ability of molecule to inhibit the P-gp function could be calculated using the ratio kai / ka0. The kia/k0a values were progressively decreased as the concentration of molecule increased as indicated in figure 3. The maximum of kia/k0a values were about 0.1-0.3. This means that molecules could inhibit P-gp function by 70-90 %. The result suggestion was consistent with report that quercetin and its glycoside derivatives inhibited proliferation of cancer cell and re-sensitize the MDR cells to pirarubicin via inhibit P-gp function (9). Methoxyflavones, including 3,5,6,7,8,3’,4’-heptamethoxyflavone, nobiletin, and tangeretin, and flavones exhibited MDR-reversing effect by increased the uptake of [3H] vincristine in MDR cancer cells. Their potency was influenced by the number and position of their methoxyl moieties (16) and [3H] also vincristine was increased uptake in Caco-2 (P-gp expression) cell as a concentration of methoxyflavone dependent manner (17). Moreover, co-incubation of plant extract with anticancer agent (typical P-gp substrate), resulting plant extract increased accumulation of anticancer agent in expressed P-gp cancer cell (18,19). Herbal extracts were found to enhance the paclitaxel sensitivity in HeLa cells via the inhibition of P-glycoprotein function. (19). Similarly, Thai plant extract increased [3H] paclitaxel uptake in the paclitaxel-resistant HepG2 as a concentration-dependent manner (18). P-gp was not only expression in cancer but it also expressed in normal cell too that was involved with drug intestinal absorption depending on the type and concentration of co-administration drugs. (20,21).
Effects of WP279, WP280 and WP283 on ROSi in K562 and K562/adr cell lines:
The effect of WP279, WP280 and WP283 on ROSi in cancer cell lines was determined that a typical experiment was shown in figure 4. The results were found that k2ROSi in both cancer cell lines was decreased by incubated with WP279, WP280 and WP283 in various concentrations for 5 min. The WP279, WP280 and WP283 could deplete k2ROSi in K562 cells by about 53 %, 55% 38% and K562/adr cells by 54%, 67%, and 58%, respectively, as shown figure 5. The esterase could be measured by k1E0. We found that before and after added WP in a cell, esterase was similar. The result suggested consistency to report that ROS produced by H2O2 treatment in acute myelogenous leukemia cell line was suppressed by methoxylated flavonoids (22) The superoxide generation induced with N-formyl-methionyl-leucyl-phenylalanine in human neutrophil was decreased by 5,7,3¢,4¢-tetrahydoxy-3-methoxyflavones, luteolin and quercetin (23). Methoxyflavone, Nobiletin and Tangeretin inhibited LPS (Escherichia coli)-induced nitric oxide production in RAW 264.7 cells by dose-dependent manner (24). Wogonin, methoxyflavonoid inhibited the oxidative neuron cell damage induced by H2O2, xanthine/xanthine oxidase and exhibited 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity. It also inhibited lipid peroxidation in rat brain homogenates (25). Similarly, Baicalein, oroxylin A and wogonin exhibited antioxidative and free radical scavenger activities. These three flavonoids also affected to decrease inflammation in in vivo study (26). Moreover, extracted compounds contained various kinds of flavonoid from natural products exhibited antioxidant property. The methanol extract of the leaves and stem of Calpurnia aurea and Buddleja saligna were effective scavenger of the ABTS and DPPH radical (27,28).
 |
Figure 1: Chemical structure of WP279, WP280 and WP283.
|
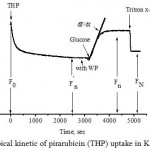 |
Figure 2: Typical kinetic of pirarubicin (THP) uptake in K562/adr cells.
|
The fluorescence intensity at λem =590 nm (lex = 480 nm) was recorded as a function of time. Cells (2×106 cells) were incubated in 2 mL HEPES Na+-buffer, pH 7.25 in the absence of glucose under continuous stirring at 37 oC in 1-cm quartz cuvette. The cells were energy depleted by incubation with NaN3 for 30 min. Pirarubicin (1 mM) was added to cells. At t=0, the initial fluorescence intensity was defined as F0 and reach at a steady state, F¢n. At the steady state, a WP molecule was added and followed by 5 mM glucose, resulting ATP was synthesized and fluorescence intensity was increased due to the efflux of pirarubicin, the fluorescence intensity was defined as Fn at the new steady state. The addition of Triton X-100 yielded the equilibrium state, giving the fluorescence intensity of FN.
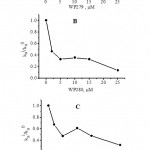 |
Figure 3: Efficacy of WP 279 (A), WP280 (B) and WP283 (C) to inhibit the P-glycoprotein-mediated pirarubicin efflux. The ratio kia/k0a was plotted as a function of the concentrations. |
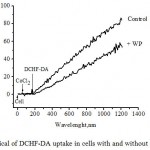 |
Figure 4: Typical of DCHF-DA uptake in cells with and without WP molecule. The fluorescence intensity at λem =523 nm (lex = 502 nm) was recorded as a function of time. |
Cells (105 cells) with and without molecule were suspended in 2 mL HEPES-Na+, pH 7.25 at 370C under vigorous stirred. Then 20 mM of CoCl2 was added. The following addition of 100 nM DCHF-DA, which led to increase in fluorescence intensity due to DCHF-DA was hydrolyzed by intracellular esterases to non-fluorescent DCHF and then DCHF reacted with ROSi to form highly fluorescent DCF.
![Figure 5: Effects of WP 279 (A), WP280 (B) and WP283 (C) on k2[ROSi] of K562 cells and WP 279 (D), WP280 (E) and WP283 (F) on k2[ROSi] of K562/adr cells.](https://www.biotech-asia.org/wp-content/uploads/2016/05/Vol_8_no1_Effe_Jira_fig5-150x150.jpg) |
Figure 5: Effects of WP 279 (A), WP280 (B) and WP283 (C) on k2[ROSi] of K562 cells and WP 279 (D), WP280 (E) and WP283 (F) on k2[ROSi] of K562/adr cells. |
Conclusions
We concluded that the present study indicated that WP279, WP280, and WP283 could partially inhibit the functionality of P-glycoprotein in drug- resistance cancer cells. WP molecules also decreased in ROSi in K562 and K562/adr cells. Due to dual property of WP molecules, they were promising to develop for anticancer agent in the future.
Acknowledgements
We would like to thanks the Center of Excellence for Molecular Imaging, Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University for their facilities and financial support. We thanks Assoc. Prof. Dr. Wilart Poompimon, Department of Chemistry, Faculty of Science, Lampang Rajabhat University for providing WP molecules.
References
- Barrand M.A., Bagrij T. and Neo S.Y., Gen. Pharmac., 28, 639, (1997).
- Choi C.H.,Cancer Cell Int.,5,30,(2005).
- Yang K., Wu J. and Li X., BioSci. Trends., 2, 137,(2008).
- Pietta P.G., J. Nat. Prod., 63,1035,(2000).
- Kothan S., Dechsupa S., Leger G., Moretti J.L., Vergote J. and Mankhetkorn S., Can. J. Physiol. Pharmacol.,82, 1084,(2004).
- Dechsupa S., Kothan S., Vergote J., Leger G. and Mankhetkorn S., Cancer Biol. Ther., 6, 56,(2007).
- Tran P.L.C.H.B., Kim S.A., Choi H.S., Yoon J.H. and Ahn S.G., BMC Cancer, 10,276,(2010).
- Shukla S. and Gupta S., Mol. Carcinog., 48, 243,(2009).
- Choiprasert W., Dechsupa N., Kothan S., Garrigos M. and Mankhetkorn S., Am. J. Pharmacol. Toxicol.,5,24,(2010).
- Kitagawa S., Nabekura T., Takahashi T., Nakamura Y. and Tsukahara G., Biol. Pharm. Bull., 28,2274, (2005).
- Rithidech K.N., Tungjai M. and Whorton E.B., Mutat. Res., 585, 96, (2005).
- Tungjai M., Poompimon W., Loetchutinat C., Kothan S., Dechsupa N. and Mankhetkorn S., Open Drug Delivery J., 2, 10,(2008).
- Meesungneon J., Jay-Gerin J.P. and Mankhetkorn S., Can. J. Physiol. Pharmacol., 80, 1054,(2002).
- Mankhetkorn S., Dubru F., Hesschenbrouck J., Fiallo M. and Garnier-Suillerot A., Mol. Pharmacol., 49, 532, (1996).
- Loetchutinat C., Kothan S., Dechsupa S., Meesungnoen J., Jay-Gerin J.P. and Mankhetkorn S., Radiat. Phys. Chem., 72, 323,(2005).
- Ohtani H., Juichi M., Naito M., Tsuruo T. and Sawada Y., Pharm. Res., 24, 1936,(2007).
- Takanaga H., Ohnishi A., Yamada S., Ohtani H. and Sawada Y., J. Pharmacol. Exp. Ther., 293, 230, (2000).
- Kawami M., Yumoto R., Nagai J., Junyaprasert V.B., Soonthornchareonnon N., Patanasethanont D., Sripanidkulchai B. and Takano M., Drug Metab. Pharmacokinet., 25,155,(2010).
- Takara K., Horibe S., Obata Y., Yoshikawa E., Ohnishi N. and Yokoyama T., Biol. Pharm. Bull., 28, 138, (2005).
- Prasad V., Jain V., and Mishra P.R., Journal of Pharmacology and Toxicology., 1,54,(2006).
- Daodee S., Wangboonskul J., Jarukamjorn K., Sripanidkulchai B. and Murakami T., Pak. J. Biol. Sci., 10, 2078, (2007).
- Jeong J.M., Choi C.H., Kang S.K., Lee I.H., Lee J.Y. and Jung H., J. Pharm. Pharmaceut. Sci., 10, 537,(2007).
- Lu H.W., Sugahara K., Sagara Y., Masuoka N., Asaka Y., Manabe M. and Kodama H., Arch. Biochem. Biophys., 393, 73,(2001).
- Choi S.Y., Ko H.C., Ko S.Y., Hwang J.H., Park J.G., Kang S.H., Han S.H., Yun S.H., and Kim S.J., Biol. Pharm. Bull., 30,772,(2007).
- Cho J. and Lee H.K., Eur. J. Pharmacol., 485, 105, (2004).
- Huang W.H., Lee A.R. and Yang C.H., Biosci. Biotechnol. Biochem., 70, 2371, (2006).
- Adedapo A.A., Jimoh F.O., Koduru S., Afolayan A.J. and Masika P.J., BMC Complementary Alternative Med., 8,53, (2008).
- Adedapo A.A., Jimoh F.O., Koduru S., Masika P.J. and Afolayan A.J. BMC Complementary Alternative Med. 9,21, (2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.





