How to Cite | Publication History | PlumX Article Matrix
M.Montazeri1, N.Rashidi2, E.Biazar3,4,*, H.Rad Gh5, M.Sahebalzamani6, S.Heidari K.3 and A.Majdi7
¹Faculty of Medical Sciences, Tehran University of Medical Sciences, Tehran Heart Center Hospital, Tehran (Iran). ²Faculty of Medical Sciences, Tehran University of Medical Sciences, Shariati Hospital, Tehran (Iran). ³Proteomics Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 4Department of Biomaterial Engineering, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran. 5Faculty of Medical Sciences, Tonekabon Branch, Islamic Azad University, Tonekabon (Iran). 6Department of Biomedical Engineering, Science and Research Branch, Islamic Azad University, Tehran (Iran). 7Young Researchers Club, Tonekabon Branch, Islamic Azad University, Tonekabon (Iran).
ABSTRACT: Tissue engineering is defined as the designing and engineering of structures to rebuild and repair a body damaged tissue. Scaffolding Poly Hydroxy Butyrate Valrate (PHBV) has shown good biocompatibility and biodegradable properties. Nanofibers have improved the performance of biomaterials, and could be considered effective. One of the important methods for designing nanofiber scaffold is the electrospinnig method. In this study, PHBV nanofibers were well designed; then, modified with the immobilized gelatin via the chemically method. The samples were evaluated by ATR-FTIR, SEM, and finally, myocardiocyte culture. ATR-FTIR structural analysis showed the presence of gelatin on the nanofiber surfaces. The SEM images showed the size average of nanofibers as to be about 280 nm; that increased with a gelatin coating up to 500 nm. Cellular investigations (myocardiocyte) showed better adhesion and cell growth and proliferation of coated samples than uncoated samples. In this work, the PHBV nanofibers with a size average about 280 nm were designed. Nanofibers were successfully coated with gelatin via the chemically methods. These gelatin-coated nanofibers could be used well for heart tissue engineering.
KEYWORDS: PHBV; Nanofiber; Gelatin Coating; Cellular Investigation; Cardiac Muscle Cells
Download this article as:| Copy the following to cite this article: Montazeri M, Rashidi N, Biazar E, Rad Gh H, Sahebalzamani M, Heidari K. S, Majdi A. Compatibility of Cardiac Muscle Cells on Coated-Gelatin Electro-Spun Polyhydroxybutyrate/valerate Nano Fibrous Film. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Montazeri M, Rashidi N, Biazar E, Rad Gh H, Sahebalzamani M, Heidari K. S, Majdi A. Compatibility of Cardiac Muscle Cells on Coated-Gelatin Electro-Spun Polyhydroxybutyrate/valerate Nano Fibrous Film. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9317/ |
Introduction
Tissue engineering is a rapidly growing area that aims to create, repair and/or replace tissues and organs by using combinations of cells, biomaterials, and/or biologically active molecules. Tissue engineering strategies promise to revolutionize current therapies for irreversible myocardial damage, heart failure, and significantly improve the quality of life for millions of patients. The most challenging goal in the field of cardiovascular tissue engineering is the creation of an engineered heart muscle. Today, unlike heart valves or blood vessels, heart muscle has no replacement alternatives. Recent advances in methods of stem cell isolation and culture in bioreactors and the synthesis of bioactive materials show promise to contribute to creation of engineered cardiac tissue in vitro [1-3].
The biomaterial scaffold plays a key role in most tissue engineering strategies. To guide the organization, growth, and differentiation of cells in tissue engineered constructs, the biomaterial scaffold should be able to provide not only a physical support for the cells but also the chemical and biological cues needed in forming functional tissues [4].
Poly Hydroxy Butyrate Valerate (PHBV) is a biomaterial that is used in a variety of applications including surgical sutures, wound dressing, drug delivery and tissue engineering. This is due to its specific properties such as good biocompatibility,biodegradablity, non toxicity as well as its piezoelectricity features. However, this material is a hydrophobic polyester which should be modified with other materials until it improves its cell adhesion and hydrophilicity properties [5-9]. It has been generally accepted that extracellular matrix mimics may improve the attachment, proliferation, and the viability of the cultured cells [10]. Electro-spinning has been rapidly developed into a technique to prepare nanofibers with the diameter ranging from tens of nanometers to several microns [11-13].The electro-spun fibrous mats also show extremely high surface area and large porosity. Besides, the fibrous structure of the electro-spun mats may mimic the extracellular matrix. It is well-known that the gelatin is a natural Biomaterial derived from the collagen inside animals skin and bones [13].Therefore; during the last few years, many works considering the tissue engineering of electro-spun nanofibers have been reported. Most recently, electro-spun nanofibers were prepared by Yang et al and were applied in neural tissue engineering [14]. Although the presented nanofibers may mimic the morphologies of extracellular matrix to some extent, some modifications are still required to create a friendly environment for the cells attachment, proliferation, and functions such as communications. Some natural materials such as collagen, fibronectin and some peptides have been reported as scaffold modifiers [15,16]. Controlling surface properties is very important for the high performance of adhesion. Biomaterials wettability is an important factor in the surface modification of materials. Surface modification of hydrophobic polymer surfaces can be achieved by wet (acid, alkali), dry (plasma) and radiation treatments (ultraviolet radiation and laser) [17-20].
In this study, the coated gelatin PHBV nanofibers were obtained through the chemical method. The samples were evaluated by ATR-FTIR, SEM and also the cell culture with Cardiac Muscle cells.
Materials and Methods
Nanofiber Preparation
A poly (3-hydroxybutyrate-co-3-hydroxyvalerate) PHBV containing 5 mol% of 3-hydroxyvalerate with 680,000 molecular weight was purchased from Sigma Chemical Co. 2, 2, 2-trifluoroethanol (TFE) to prepare PHBV solution was also purchased from Sigma-Aldrich Chemicals and was used as received without further purification. An electro-spinning apparatus used in this study was prepared from the Asia Nanomeghyas Company (Iran). The PHBV was dissolved at determined concentration in TFE. The PHBV solution (2%w) in a glass syringe was controlled by the syringe pump. A positive high voltage source through a wire was applied at the tip of the syringe needle. In this situation, a strong electric field was generated between the PHBV solution and a collector. When the electric field reached a critical value with increasing voltage, the mutual charge repulsion overcame the surface tension of the polymer solution and the electrically charged jet was ejected from the tip of a conical shape as the Taylor cone. Ultrafine fibers were formed by the narrowing of the ejected jet fluid as it underwent increasing surface charge density due to the evaporation of the solvent. The electro-spun PHBV nanofibrous mat was carefully detached from the collector and was dried in vacuo for 2 days at room temperature to remove solvent molecules completely. The used parameters for this nanofibers preparation can be seen in Table1.
Table 1: Used parameters for nano fibers preparation.
| Time (h) | Temperature(°C) | Voltage(kv) | Syringe tip distance to deram (mm( | Injected speed)mL/min( | Dram speed (rpm ) | Syringe Diameter(mm) | |
|
7 |
30 |
20 |
75 |
2 |
1000 |
17 |
|
Gelatin Immobilization
Gelatin (Sigma) was immobilized onto the nanofiber surface based on the following protocol. The nanofibrous mat was submerged into the Gelatin solution (10 mg/mL in distilled water solution) and was shaken gently for 2 h at 40 °C. The samples were exposured to glutaraldehyde 12% in hot water bath. The obtained samples were placed inside a vacuum oven so that they would fully lose humidity.
Fourier transmission infrared spectroscopy
The samples were examined by FTIR (Bruker-Equinox 55; Bruker Optics, Billerica, MA) before and after adjustment. The samples were scratched into powder and were produced as capsules using KBr, and then were investigated.
Scanning electron microscopy
The surface characteristics of various modified and unmodified films were studied by scanning electron microscopy (SEM; Cambridge Stereo-scan, model S-360; Cambridge Instruments, Wetzlar, Germany) to analyze the changes in the surface morphology. The films were first coated with a gold layer (Joel fine coat, ion sputter for 2 hours) to provide surface conduction before their scanning.
Cellular analysis
Primary cultures can be initiated from cardiac muscle tissues of newborn rats as described below.
Digest minced cardiac muscle tissue with 1% trypsin (gibco) in Ca2+ and Mg2+ free PBS for 20 min at 37°C. Satellite cells can also be isolated by digesting minced adult muscle with 0.2% (w/v) collagenase (Roche) in HBSS followed by 0.1% (w/v) trypsin in PBS. Inactivate the trypsin with an equal volume of 0.1% soya bean trypsin inhibitor (Sigma) in PBS. Cells enzymatically released from muscle tissue usually contain a significant number of fibroblasts in addition to myogenic cells.
Separate fibroblasts from myogenic cells using differential adhesion: incubate cells for 30 min on non-coated plastic culture dishes, and collect the non-adherent muscle Repeat this differential cell adhesion step.
Plate myoblasts on to gelatin-coated dishes in Eagle’s minimal essential medium (MEM) (Life Technologies) supplemented with 10% horse serum (Hyclone) and 2% embryo extract (Difco). It is advisable to add ascorbic acid (Sigma) to 100 Il-M to enhance collagen production.
Myoblasts enter the post-mitotic G0 phase and myoblast fusion (fusion- burst) becomes evident within 48 h after plating. Around the time of fusion-burst, transcription of muscle-specific genes is up-regulated. The activation of muscle-specific genes is also observed in fusion-arrested myoblasts in low calcium medium. Myofibrillogenesis takes place in multinucleated myotubes and spontaneous twitching can be observed within 7 days of plating. Aliquots of cell suspension in RPMI medium including 300,000 cardiomyocyte cells were seeded on a 6-multiwell cell culture plate (Orange County Industrial Plastics) which was precoated with samples. The film was put in an incubator (37°C, CO2) over three hours for cell attachment, followed by rinsing of the loosely attached cells with phosphate buffer solution, and adding 2 mL of fresh medium to the cell culture in the incubator for seven days. Proliferation of cells was determined from measure-ment of viable cell numbers by MTT assay. The MTT tetrazolium compound was reduced by living cells into a colored formazan product that was soluble in tissue culture medium. The quantity of formazan product was directly proportional to the number of viable cells in the culture. The assays were performed by adding 1 mL of MTT solu-tion (Sigma-Aldrich) and 9 mL of fresh medium to each well after aspirating the spent medium, and incubating at 37°C for four hours with protection from light. The colorimetric measurement of formazan dyeing was performed at a wavelength of 570 nm using a microplate reader (Rayto,Shenzhen, People’s Republic of China).
Results
FTIR Results
The results, from the ATR-FTIR spectrum of the uncoated nanofiber sample and the nanofiber sample modified with Gelatin, have been shown in figure 1. In figure 1a, the strong band in 1722 cm-1 has been shown to be related to the C = O group. The stretching band in 800-975 cm-1 has been shown to be related to the- C-O-C- group and the stretching band in 2900-3000 cm-1 has been shown to be related to the -CH3 groups. Figure 1b also shows the strong band in 1722 cm-1 as related to the C=O group , the stretching band in 800-975 cm-1 as related to the -C-O-C- group and the stretching bands in 2800-3000 cm-1 as related to the -CH3 groups. The stretching band in 3400 cm-1 and 3761 cm-1 are related to the OH and NH groups due to the presence of gelatin.
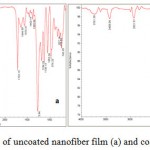 |
Figure 1: FTIR analysis of uncoated nanofiber film (a) and coated nanofiber film (b). |
SEM Investigations
The figures 2 and 3 show the Electron Microscopy Images of the uncoated and the coated nanofibers with gelatin in different magnifications. Figure shows the nanofiber mat prepared to the electro-spinning method of different magnifications (2a ; 5000x – 2b; 20000x).
 |
Figure 2: SEM images of PHBV nanofibers in different magnifications (2a ; 5000x – 2b; 20000x.) |
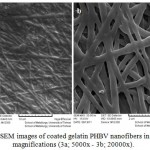 |
Figure 3: SEM images of coated gelatin PHBV nanofibers in different magnifications (3a; 5000x – 3b; 20000x). |
Figure 3 shows the coated nanofiber mat prepared to the electro-spinning method of different magnifications (3a; 5000x – 3b; 20000x).
Image analysis of the electrospun normal nanofibers fabricated from 2wt% PHBV-TFE solution and coated nanofiber revealed an unimodal distribution of fiber diameters with an observed average diameter of 280 nm and 500 nm respectively (Figure 4).
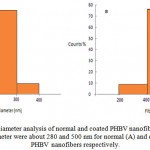 |
Figure 4: Fiber diameter analysis of normal and coated PHBV nanofibers. The average fiber diameter were about 280 and 500 nm for normal (A) and coated (B) PHBV nanofibers respectively. |
Cellular Study
Figure 5 shows the MTT assay for TCPS (control), the uncoated nanofiber and the coated nanofiber samples. The results showed a high viability for the samples of the uncoated nanofiber and the coated nanofiber (110, 132 % respectively), but the coated nanofiber showed a better viability than the uncoated nanofiber. Also, these samples caused more cells to proliferate. Figure 6 showed images of the cell culture on the coated nanofiber and the control sample. The image a is related to the control sample and the image b is related to the the coated nanofiber. Cellular images showed well growth in the vicinity of the coated nanofiber.
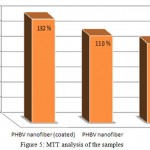 |
Figure 5: MTT analysis of the samples.
|
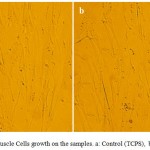 |
Figure 6: Cardiac Muscle Cells growth on the samples. a: Control (TCPS) , b: Coated nanofiber . |
Discussion
In this study, the PHBV nanofibers with a size average about 280 nm were designed. Nanofibers were successfully coated with gelatin via the chemical methods shown in the analysis The smooth and homology modified nanofibers have been clearly shown in the figures. The size average was obtained for the modified nanofibers to be about 500 nm, whose increasing is due to gelatin coated on the PHBV surfaces. The results showed a high viability for the samples of the gelatin coated nanofibers. Also, these samples caused more cells to proliferate. Cellular images showed well growth in the vicinity of nanofibers especially the coated nanofiber. These gelatin coated nanofibers could be used well for heart tissue engineering
References
- Akhyari, P., Fedak, P. W., Weisel, R. D., Lee, T. Y., Verma, S., Mickle, D. A., et al: Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation 2002, 106: I137– I142.
- Akins, R. E., Boyce, R. A., Madonna, M. L., Schroedl, N. A., Gonda, S. R., McLaughlin, T. A., et al: Cardiac organogenesis in vitro:reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng 1999,5, 103– 118.
- Anversa, P., Sussman, M. A., & Bolli, R: Molecular genetic advances in cardiovascular medicine: focus on the myocyte. Circulation 2004, 109, 2832– 2838.
- Langer, R., & Tirrell, D. A. (2004). Designing materials for biology and Nature 428, 487–492.
- Williams SF, Martin DP, Horowitz DM, Peoples OP: PHA applications: addressing the price performance issue: I. Tissue engineering Int. Biol. Macromol 1999,25:111
- Liu J, Zhao B, Zhang Y, Lin Y, Hu P, Ye C: PHBV and predifferentiated human adipose-derived stem cells for cartilage tissue engineering. J Biomed Mater Res A 2010,94(2):603-610
- Meng W, Kim SY, Yuan J, Kim JC, Kwon OH, Kawazoe N, Chen G, Ito Y, Kang IK: Electrospun PHBV/collagen composite nanofibrous scaffolds for tissue engineering. J Biomater Sci Polym Ed 2007,18(1):81-94
- Ndreu A, Nikkola L, Ylikauppila H, Ashammakhi N, Hasirci V: Electrospun biodegradable nanofibrous mats for tissue engineering. Nanomedicine (Lond) 2008,3(1):45-60
- Torun Köse G , et al:Tissue engineering of bone using collagen and PHBV matrices . Technology and Health Care 2002,10: 3-4
- Gerard C, Catuogno C, Amargier-Huin C, Grossin L, Hubert P, Gillet P, Netter P, Dellacherie E, Payan E: The effect of alginate, hyaluronate and hyaluronate derivatives biomaterials on synthesis of non-articular chondrocyte extracellular matrix. J Mater Sci Mater Med 2005,16(6):541-551
- Li D , Xia YN: Electrospinning of nanofibers: Reinventing the wheel? . Advanced Materials 2004,16: 1151-1170
- Huang ZM, Zhang Y Z, Kotaki M and Ramakrishna S: A review on polymer nanofibers by electrospinning and their applications in nanocomposites . Sci. Technol 2003,63:2223
- Smith LA and Ma PX: Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004,39:125
- Yang F, Murugan R, Wang S and Ramakrishna S: Electrospinning of Nano/micro Scale Poly(L-lactic acid) Aligned Fibers and their Potential in Neural Tissue Engineering.Biomaterials 2005, 26 : 2603
- Ito T, Nakamura T, Suzuki K, Takagi T, Toba T, Hagiwara A, Kihara K, Miki T, Yamagishi H, Shimizu Y:Regeneration of hypogastric nerve using a polyglycolic acid (PGA)-collagen nerve conduit filled with collagen sponge proved electrophysiologically in a canine model. J. Artif. Organs 2003,26: 245
- Kapur TA and Shoichet MS: Chemically bound nerve growth factor for neural tissue engineering applications. J Biomater Sci: Polymer Ed 2003,14:383–394
- Lu HW, Lu QH, Chen WT, Xu HJ and Yin J : Cell culturing on nanogrooved polystyrene petri dish induced by ultraviolet laser irradiation . Materials Letters 2004, 58(1-2):29-32
- Roucoules V, Gaillard F, Mathia TG and Lanteri P : Hydrophobic mechano chemical treatment of metallic surfaces.Wettability measurements as a means of assessing homogeneity.in Colloids And Interf. Sciences 2002,97(1-3):177-201
- Hay KM, Dragila MI, Liburdy J: Theoretical model for the wetting of a rough surface . of Colloid Interf. Science 2008,325:472–477
- Kogelschatz U: Dielectric – barrier discharges: their history , discharge physics and industrial applications. Plasma chem. Plasma 2003,23:1-46

This work is licensed under a Creative Commons Attribution 4.0 International License.





