How to Cite | Publication History | PlumX Article Matrix
Does Chip Size of the Lignocellulosic Bagasse Influence Lignin Degradation with NaOH treatment
C. Soundarrajan1*, S. John Vennison2, K. Saraswathi3 and E. S. Challary Emmanuel4
1Corrosion Protection Division, Central Electrochemical Research Institute Karaikudi - 630 006 India.
2Department of Biotechnology, Anna University of Technology, Tiruchirappalli - 620 024 India.
3Department of Botany, Thiagarajar College, Madurai - 625 009 India.
4Department of Microbiology, St. George College, Bangalore - 560 033 India.
Corresponding Author E-mail: soundarbiotech@gmail.com
ABSTRACT: Cellulose is a linear polymer of glucose in plants. It is associated with hemicelluloses and other structural polysaccharides, and surrounded by a lignin seal. The lignin encapsulation prevents enzymes and acids from accessing some regions of the cellulose polymers. Treatment of lignocellulosic wastes increases the accessible surface area of cellulose and enhances conversion of the cellulose to glucose. In present study, sieve analysis was carried out in order to study the optimum size of ligno cellulosic material required for the effective degradation of lignin. The lignocellulosic waste namely bagasse was powdered and sieved through -22,-30,-44 and -52 mesh sizes using sieve shaker and then treated with 2% NaOH. The efficacy of the treatment was analyzed by FTIR spectroscopy.
KEYWORDS: Cellulose; Lignin; Lignocellulose; FTIR
Download this article as:| Copy the following to cite this article: Soundarrajan C, Vennison S. J, Saraswathi K, Emmanuel E. S. C. Does Chip Size of the Lignocellulosic Bagasse Influence Lignin Degradation with NaOH treatment. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Soundarrajan C, Vennison S. J, Saraswathi K, Emmanuel E. S. C. Does Chip Size of the Lignocellulosic Bagasse Influence Lignin Degradation with NaOH treatment. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9620 |
Introduction
Cellulose is produced by plants. It is a linear homopolymer of (1-4)-linked β-D-glucopyranose units. The basic repeating unit is the disaccharides cellobiose. Cellulosic materials are particularly attractive because of their relatively low cost and plentiful supply. It has served mankind as a construction material such as wood, textile fibers and in the form of paper and board.
Cellulose is also a versatile starting material for chemical conversions as well as a variety of stable cellulose derivatives used in many areas of industry and domestic life1. It is soluble in a few solvents and does not melt without thermal degradation because of the secondary and tertiary conformation of cellulose, as well as its close association with lignin, hemicellulose, starch, protein and mineral elements makes cellulose hydrolysis-resistant molecule2. The physical and chemical transformations of hydrogen bonds in cellulose substrates can be used for various applications. Pretreatment processes can improve the hydrolysis of lignocellulosic waste by removal of lignin and hemicellulose, reduction of cellulose crystallinity and increase the porosity3.
Pretreatments may be physical, chemical or biological which should avoid the degradation or loss of carbohydrate, to avoid the formation of inhibitory byproducts and cost-effective. The characterization of crystalline cellulose structure can be done by X-ray diffraction (XRD), Nuclear magnetic resonance (NMR), Fourier Transform Spectroscopy (FTIR), Calorimetry etc. Though X-ray diffraction gives qualitative information about hydrogen bonding and spatial conformations between cellulose molecules, FTIR gives some useful information related to the change of hydrogen bonding during crystal transformation. Modification leads to changes in the solubility, properties and behaviour of the polymer. Industrially mechanical powdering of cellulosic wastes results in various chip sizes. Chip sizes may influence in the efficiency of the treatments. Many chemicals such as ammonia, HCl, H2O2, sulfuric acid, phosphoric acid etc can be used for lignin degradation and so far there was no authentic evidence for the association of lignocellulosic chip size on lignin degradation. Thus in present study an attempt was made to treat lignocellulosic waste namely bagasse based on sieve analysis with 2% NaOH.
Materials and Methods
Sample collection
Cellulosic waste (bagasse) was collected from the sugar industry near Madurai district, Tamil Nadu, India.
Qualitative test for cellulose
Bagasse substrate solution (2ml) was placed in the test tubes and 2 drops of molisch reagent (A solution of α-napthol in 95% ethanol) was added. The solution was then poured slowly into a tube containing 2ml of concentrated sulphuric acid to observe the presence of cellulose4.
Sieve Analysis
Cellulosic waste namely bagasse was powdered and separated based on the mesh size such as -22, -30,-44 and -52 5. The sign “-” before the sieve mesh indicates the particles pass through the sieve. If a material is described as -44 meshes, then 90% or more of the powdered material will pass through a 44 mesh sieve.
Sodium Hydroxide treatment
Sodium hydroxide treatment was performed with 2% NaOH solution. Ten grams of sieve analyzed cellulosic wastes were mixed with 100 ml of the NaOH solution separately and stirred at room temperature for 2 hrs. It was then neutralized with distilled water6.
Fourier Transform Infrared Spectroscopy (FTIR) analysis
Treated cellulosic wastes 2mg were prepared by mixing with 200 mg of spectroscopic grade KBr. FTIR spectra were recorded using a Nicolet 520P spectrometer with detector at 4cm-1 resolution and 20 scans per sample7.
The overall schematic of the experiment is shown in Fig: 1.
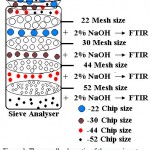 |
Figure 1: The overall schematic of the experiment.
|
Results and Discussion
Qualitative test for cellulose
Bagasse was qualitatively analyzed for the presence of cellulose is shown in Figs: 2 (a & b). Molisch test is a sensitive chemical test for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid to produce an aldehyde, which condenses with two molecules of phenol (usually α-naphthol, though other phenols (e.g. resorcinol, thymol) also give colored products) resulting in a red- or purple-coloured compound. Hexoses such as celluloses are dehydrated to 5-hydroxymethylfurfural which will condense with two molecules of naphthol to form a purple-colored product. Purple colour confirmed the presence of cellulose in the bagasse4.
 |
Figure 2: Qualitative tests for cellulose presence (a) Pure cellulose powder (Himedia) (b) Bagasse.
|
FTIR analysis of cellulosic substrates
Bagasse treated with 2% NaOH degraded into cellulose and lignin shown in Fig: 3. The treated lignocellulosic waste was analyzed using FTIR spectroscopy. The FTIR spectroscopy is an appropriate technique to establish the variations introduced by the different treatment on the chemical structures of cellulose. Pure cellulose powder (Himedia) was considered as the control for untreated and treated bagasse.
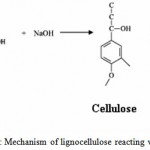 |
Figure 3: Mechanism of lignocellulose reacting with NaOH.
|
The FTIR spectrum for pure cellulose (Himedia) and untreated cellulosic waste namely bagasse was represented in Fig. 4. The FTIR spectrum for each of the four mesh sizes (-22,-30,-44,-52) for 2% NaOH was represented in Fig. 5. All spectra were dominated by the peaks ranging at 3356 and 1058 cm-1 that correspond to the stretching vibration of O-H in cellulose and C-O in hemicelluloses and cellulose, respectively. Peaks observed at 1162 cm-1 and 1640 cm-1 were indicative of the C=O bonds of hemicelluloses and confirmed the presence of hemicelluloses 8,9. The absorption at 1730 cm–1 could correspond to the acetyl and uronic ester groups of residual hemicelluloses or to the ester linkage of carboxylic group of the ferulic and p-coumaric acids of lignin10. Peaks observed at 1252 cm-1 also show the presence of hemicelluloses11. It is also reported that the absorbance from 1500 to 1599 cm-1 is associated with aromatic skeletal vibration in lignin. Similarly it was reported that the digestibility of NaOH-treated hardwood increased from 14% to 55% with the decrease of lignin content from 24–55% to 20% 12. The dry matter digestibility of lignocellulosic waste, bagasse pith increased from 27.64 to 72.86 and 61.99 percent when it was treated with 3.5 per cent sodium hydroxide or 2.5 per cent calcium hydroxide respectively13. The other chemicals normally used for pretreatments are dilute NaOH, ammonia, HCI, H2O2, sulfuric acid and phosphoric acid. Dilute NaOH pretreatment was also effective for the hydrolysis of straws with relatively low lignin content of 10–18%14 and no effect of dilute NaOH pretreatment was observed for softwoods with lignin content greater than 26%12. Whereas oxidizing agents, such as hydrogen peroxide penetrate the cellulose causing a structural change and oxidize the polymer15. Untreated bagasse showed the presence of lignin peak at 1513.22 cm-1. The peak values ranging from 1500 to 1599 cm-1 was absent in all treated bagasse. Thus dilute NaOH treatment showed good removal of lignin content in bagasse.
 |
Figure 4: FTIR spectra for pure cellulose and untreated bagasse (a) Pure cellulose (Himedia)(b) Untreated bagasse.
|
 |
Figure 5: FTIR spectrums for bagasse treated with 2% NaOH (a) -22, (b) -30, (c) -44, (d) -52 mesh sizes.
|
Conclusions
2% NaOH was found to show better removal of lignin content. Moreover it is easily neutralizable, cost effective and it also retains the structural conformation of cellulose. The FTIR spectra showed more or less same peak values in all sieve sizes for 2% NaOH treatment. Hence it was inferred that 2% NaOH treatment was found be efficient on cellulosic wastes and chip sizes do not influence the efficiency of NaOH chemical pretreatment. These treated bagasse wastes can be used as a substrate for bioethanol production using cellulose utilizing microorganisms.
Acknowledgement
The authors would like to express their thanks to St.Joseph College, Tiruchirappalli for FTIR analysis of the cellulosic samples.
References
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive cellulose chemistry: I. Fundementals and analytical methods. Wiley-VCH (1998).
- Zaldivar, J.; Nielsen,J.; Olsson,L. Appl Microbiol Biotechnol. 56 (2001) 17–34.
- McMillan, J.D.; Himmel, E. Handbook on bioethanol: production and utilization, 566 (1994) 292-324.
- Baur R D, Campbell J A,. Loescher R L, Prentice Hall N J, 1980.Carbohydrate Identification. Laboratory Manual for Chemistry for the allied health science, P136.
- De Sousa, M.V.; Monteiro, S.N.; d’Almeida, J.R.M. Poly. 23 (2004) 253-258.
- Abraham, M.; Kurup, M. Biochem. Biotechnol. 62 (1996) 201-211.
- Hinterstoisser, B.; Salmean, L. Vibrational Spectroscopy. 22 (2000) 111–118.
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, F.; Baird, M.S. Carbohydr. Res. 340 (2004) 97–106.
- Ganan, P.; Cruz, J.; Garbizu, S.; Arbelaiz, A.; Mondragon, J Appl. Polym. Sci. 94 (2004) 1489–1495.
- Sun, X.F.; Xu, F., Sun, X.F.; Xiao, B.; Sun, R.C.; Polym. Degrad. Stab. 88 (2005) 521–531.
- Sain, M.; Panthapulakkal, S. Ind. Crops Prod. 23 (2006) 1–8.
- Millet, M.A. Cellulase as a chemical and energy resource. Presentation at NSF Seminar: (1974) 25–27.
- Bjerre, A.B.; Olesen, A.B.; Fernqvist, T. Biotechnol. Bioeng. 49 (1996) 568–577.
- Tahira, F.; Khan, A.D.; Shah, A.F. Journal of Islamic Academy of Sciences, 2 (198) 89-92.
- Fan, L. T.; Gharpuray, M.M.; Lee, Y.H., Monograph, 3 (1987) Springer, Berlin.

This work is licensed under a Creative Commons Attribution 4.0 International License.





