How to Cite | Publication History | PlumX Article Matrix
Mohammad Sadeghi1*, Nahid Ghasemi¹ and Mojgan Yarahmadi²
¹Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak Iran. ²Department of English, Faculty of Humanities, Arak Branch, Islamic Azad University, Arak Iran.
ABSTRACT: The present work focused on the design of drug delivery system (DDS) based on a pH-sensitive hydrogel. The hydrogels were prepared via graft copolymerization of 2-acrylamido-2-methylpropanesulfonic acid (AMPS) monomer onto collagen backbones by a free radical polymerization technique. Sodium bicarbonate (NaHCO3) was added to function as a foaming agent under acidic conditions, rendering the hydrogels to be porous. Porous structure of hydrogel was essential in this system to yield a large surface area so that Acetaminophen release could be facilitated. The hydrogel thus prepared possessed a porous structure as determined by scanning electron microscopy. Due to the reversible swelling behavior of the hydrogels, the synthesized networks can sense the environmental pH change and achieve an oscillatory release pattern. The concentration of released Acetaminophen loaded was monitored at 266 nm on the UV spectrophotometer. Water absorption of the hydrogel could be switched on and off swiftly by control of pH of the surrounding environment. Therefore, the synthesized hydrogels in this work can be used as a drug delivery system, and that the drug release can be controlled by the pH of solution.
KEYWORDS: Collagen; Hydrogel; Acetaminophen; Releasing drug
Download this article as:| Copy the following to cite this article: Sadeghi M, Ghasemi N, Yarahmadi M. Drug Release Study from Superabsorbent Hydrogel Based on Poly 2-acrylamido-2-methylpropanesulfonic Acid Grafted Collagen. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Sadeghi M, Ghasemi N, Yarahmadi M. Drug Release Study from Superabsorbent Hydrogel Based on Poly 2-acrylamido-2-methylpropanesulfonic Acid Grafted Collagen. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9234/ |
Introduction
The market for superabsorbent polymers (SAPs) has increased by a factor of 5 over the past 10 years. These materials are crosslinked hydrophilic polymers, capable of absorbing large quantities of water, saline or physiological solutions.1,2 The absorbed fluids are hardly removable even under some pressure. They are widely used in various applications such as hygienic, foods, cosmetics, and agriculture.2-4This accounts for increase in the worldwide production of superabsorbent polymers (SAPs) from 6000 tons in 1983 to 450000 tons in 1996.1 Nowadays, the worldwide production of SAPs is more than one million tons in year. Hence, synthesis and investigation of specific and new superabsorbent hydrogels with high absorbency, mechanical strength and initial absorption rate, is the main goal of the several research groups in the world.5-10.The properties of the swelling medium (e.g. pH, ionic strength and the counter ion and its valency) affect the swelling characteristics. SAPs responding to external stimuli such as heat, pH, electric field, chemical environments, etc, are often referred to as “intelligent” or “smart” polymers. Among these, pH-sensitive hydrogels have been extensively investigated for potential use in site-specific delivery of drugs to specific regions of the gastrointestinal tract and have been prepared for delivery of low molecular weight protein drugs. Therefore, these hydrogels have important applications in the field of medicine, pharmacy, and biotechnology11,12. Drug release from solid matrices systems, made of polymer(s) and drug(s), is a basic concept for studies on controlled drug delivery. the most interesting class of polymers in this application is given by hydrogels, also pH-sensitive ones. Hydrogels are special soft and pliable polymeric materials that can be absorb large quantities of water, saline or physiological solutions while the absorbed solutions are not removable even under pressure. In the swollen state, these become soft and rubbery, resembling a living tissue and some possess excellent biocompatibility. Thus, polymeric hydrogels are of considerable interest as biomaterials in drug delivery research.
Experimental
Materials
Hydrolyzed collagen (ParvarNovin-E Tehran Co.) was industrial grade which is available in market and has nearly 25% insoluble phosphate salt. 2-acrylamido-2-methylpropanesulfonic acid (Merck, Darmstadt, Germany), N’,N’-methylene bisacrylamide and ammonium persulfate (Fluka, Buchs, Switzerland) were of analytical grade and used without further purification. The drug, Acetaminophen, was obtained from JaberebneHayan Pharmaceutical Co. (Tehran, Iran). The chemical structure of drug is shown in Figure 1. Double distilled water was also used for the hydrogel preparation and swelling measurements.
Preparation of hydrogel
A pre-weighed amount of hydrolyzed collagen (1.0-4.0 g) was dissolved in 40 mL degassed distilled water and filtered to remove its insoluble salt. The solution was added to a 1-L three-neck reactor equipped with a mechanical stirrer (RZR 2021, a three-blade propeller type, Heidolph, Schwabach, Germany) and the reactor was immersed in a thermostated water bath preset at a desired temperature (65 oC). Then 2-acrylamido-2-methylpropanesulfonic acid (2.0-8.0 g) was added to the reactor. After stirring for 10 min, ammonium persulfate (0.01-0.40 g APS in 5 mL H2O) and methylene bisacrylamide (0.05-0.20 g in 5 mL H2O) were added simultaneously to the reaction mixture. The temperature was maintained at 65 oC and the reaction mixture was stirred continuously (300 rpm) for 1 h. At the end of the propagation reaction, the gel product was poured into ethanol (200 mL) and was dewatered for 12 h. Then, the product was cut into small pieces, washed with 200 mL ethanol and filtered. The particles were dried in an oven at 50 oC for 12 h. After grinding, the powdered superabsorbent hydrogel was stored in absence of moisture, heat and light.
Determination of drug loading
Loading model drug into a hydrogel was performed using a contact adsorption technique. The vacuum dried powdered samples (1±0.0001 g), with average particle sizes between 40 and 60 mesh (250–350 ), were accurately weighted and immersed in the aqueous solution of drug (0.6 g dissolved in 50 mL distilled water) at 0oC for 25h to reach the equilibrated state. The swollen hydrogels loaded with drug were placed in a vacuum oven and dried under vacuum at 37oC.
The amount of drug content entrapped in the hydrogels was determined by an indirect method. After the gel preparation, the washings were collected, filtered with a 0.45 Millipore filter and tested at lmax 266 nm using UV/VIS spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan).
The drug entrapped exhibited the same lmax as the free drug. This clearly indicates that the drugs entrapped have not undergone any possible chemical reaction during the matrix formation. The difference between the amount of drug initially employed and the drug content in the washings is taken as an indication of the amount of drug entrapped. …(1)
Standard absorbance curve
The standard calibration curve of the absorbance as a function of drug concentration was studied at 266 nm on the UV spectrophotometer.
In vitro drug release
The samples (0.1±0.0001 g) were immersed into 50 mL of the release medium (simulated gastric and intestinal fluids, SGF and SIF) with different pH values (pH 1.2 or 7.4) at 37oC with agitation. At given time intervals, 1 mL of the release medium was removed using a syringe attached with a 0.45 Millipore filter and after suitable dilution the concentration of released drug was measured by UV spectrophotometer at 266 nm 9-11. The drug release percent was calculated twice using the following equation: …(2)
where L and Rt represent the initial amount of drug loaded and the final amount of drug released at time t.
Results and Discussion
Synthesis and Mechanism
The mechanism for crosslinking graft copolymerization of AMPS onto collagen backbones in the presence of APS and MBA is shown in Scheme 1. In the first step, the thermally dissociating initiator, i.e. APS, is decomposed under heating (80 oC) to produce sulfate anion-radicals. Then, the anion-radicals abstract hydrogen from one of the functional groups (i.e. COOH, SH, OH, and NH2) in side chains of the collagen backbones to form corresponding macro-initiators. These macroradicals initiate grafting of AMPS onto collagen backbones leading to a graft copolymer. Crosslinking reaction also occurred in the presence of the crosslinker, i.e. MBA.
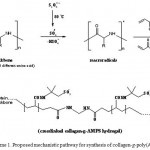 |
Scheme 1: Proposed mechanistic pathway for synthesis of collagen-g-poly(AMPS) hydroge. |
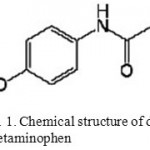 |
Figure 1: Chemical structure of drug Acetaminophen. |
Scanning electron microscopy
One of the most important properties that must be considered is hydrogel microstructure morphologies. Figure 2 shows the scanning electron microscope (SEM) photographs of the surface (Fig. 2A) and the cross-sectional area (Fig. 2B) of the hydrogel with interconnected pores. These pictures verify that the synthesized polymer in this work have a porous structure, where the pores might be induced into the hydrogel by water evaporation resulting from reaction heat. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers. The cross-sectional view of hydrogels (Fig. 2B) also exhibited large, open, channel-like structure.
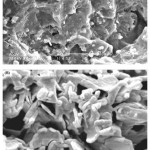 |
Figure 2: SEM photograph of the optimized superabsorbent hydrogel (A) Surface of porous hydrogel; (B) Cross-sectional area of porous hydrogel. |
pH-Sensitivity and Pulsatile Behavior
Equilibrium swelling studies indicated that the ionic hydrogels were sensitive to environmental pH. Therefore, in this series of experiments, swelling ratio for the synthesized hydrogels was measured in different pH solutions ranged from 1.0 to 13.0 (Fig. 3). Since the swelling capacity of all “anionic” hydrogels is appreciably decreased by addition of counter ions (cations) to the swelling medium, no buffer solutions were used. Therefore, stock NaOH (pH 10.0) and HCl (1.0) solutions were diluted with distilled water to reach desired basic and acidic pHs, respectively. Maximum swelling (98 g/g) was obtained at pH 9. In acidic media, the most of carboxylate groups are protonated, so decreased repulsion of anionic groups leads to a decreased swelling ratio.
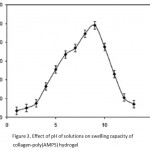 |
Figure 3: Effect of pH of solutions on swelling capacity of collagen-poly(AMPS) hydrogel.
|
At higher pHs (5-9), some of carboxylate groups are ionized and the electrostatic repulsion between COO- groups causes an enhancement of the swelling capacity. The reason of the swelling-loss for the highly basic solutions is “charge screening effect” of excess Na+ in the swelling media which shield the carboxylate anions and prevent effective anion-anion repulsion.
pH-Reversibility for Collagen-poly(AMPS) Hydrogel
Since the hydrogels show different swelling behaviors at various pHs, we investigated their pH-reversibility in the solutions buffered at pHs 2.0 and 9.0 (Figure 4). The figure shows a stepwise reproducible swelling change of the hydrogel at 35 oC with alternating pH between 2.0 and 9.0.
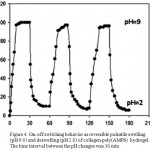 |
Figure 4: On-off switching behavior as reversible pulsatile swelling (pH 9.0) and deswelling (pH 2.0) of collagen-poly(AMPS) hydrogel. The time interval between the pH changes was 30 min. |
At pH 9.0, the hydrogel swells up to 98 g/g due to anion–anion repulsive electrostatic forces, while, at pH 2.0, it shrinks within a few minutes due to protonation of carboxylate groups. This sharp swelling–deswelling behavior of the hydrogels makes them suitable candidates for controlled drug delivery systems. Such on-off switching behavior as reversible swelling and deswelling has been reported for other ionic hydrogels in figure 5 12-13.
Standard calibration curve
The calibration curve of the absorbance as a function of the metronidazole concentration at 266 nm, shown in Figure 6, has a linear relationship with a correlation coefficient (r) of 0.979 and 0.995 at pHs 1.2 and 7.4, respectively.
 |
Figure 5: Schematic presentation swelling of poly collagen-g-poly(AMPS) hydrogel in acidic and alkalia media. |
 |
Figure 6: The standard calibration curve of the absorbance as a function of Acetaminophen concentration at 266 nm on the UV spectrophotometer at pH 1.2 (a) and pH 7.4 (b). |
In vitro AcetaminophenRelease in the Simulated Human Gastrointestinal System
In order to study potential application of collagen-based superabsorbent containing Acetaminophen as a pharmaceutically active compound, we have showed the drug release behavior of the polymers under physiological conditions in figure 7.
 |
Figure 7: Schematic presentation of poly collagen-g-poly(AMPS) hydrogel employed for encapsulation and release of Acetaminophen drug. |
The percent of released drug from polymeric carriers as a function of time is shown in Figure 8. The concentration of Acetaminophen released at selected time intervals was determined by UV spectrophotometer. The results from the present study indicate that Acetaminophen-loaded hydrogel with high degrees of drug loading (loading efficiency 81%) can be prepared by a swelling-diffusion method. The amount of Acetaminophen released in a specified time from the collagen-based hydrogel decreased when the pH of the dissolution medium was lowered (Fig. 8), suggesting final release in a medium with pH much higher than that of the stomach. At low pH values, electrostatic repulsion between the carboxylic acid groups of backbone is low, thus decreases gel swelling and minimizes release of Acetaminophen via diffusion. However, in alkaline media the presence of excess OH> increases the electrostatic repulsion between carboxylate groups, thus increases the gels swelling degree, so the release of Acetaminophen increased 13-14.
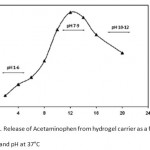 |
Figure 8: Release of Acetaminophen from hydrogel carrier as a function of time and pH at 37°C. |
The release rate experiments were also performed in SFG (pH 1.2) and SIF (pH 7.4) solutions at 37 oC (Figure 9). As can be seen from Figure 9, when pH of the medium is 1.2, the cumulative release ratio of Acetaminophen from the test hydrogels is below 16% at the end of the experiment (24 h), whereas almost 80% of the loaded drug is released within 15 h in pH 7.4 medium. Again, these results indicate that the higher swelling ratios of the hydrogel create larger surface areas to diffuse the drug. In basic solutions (pH 7.4), the electrostatic repulsion between COO- anions of grafted poly (sodium acrylate) on the hydrogel accelerates the release of Acetaminophen from the hydrogel 9.
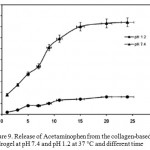 |
Figure 9: Release of Acetaminophen from the collagen-based hydrogel at pH 7.4 and pH 1.2 at 37 °C and different time. |
Figure 10 shows the effect of porosity of the hydrogel on Acetaminophen release. When compared to the dense hydrogel, the porous hydrogel provided a much faster Acetaminophen release. An initial burst release of 63 ìg (71%) of Acetaminophen from the porous hydrogel was observed during the first 12 h of experiments, followed by a continuous release of 88 ìg (94%) of Acetaminophen for up to 20 days 8,15. On the other hand, the hydrogel with dense structure showed a much lower initial burst Acetaminophen release, followed by a slower Acetaminophen release for up to 10 h. It should be pointed out that a dense structure would allow Acetaminophen release to occur primarily at the surface. In this regard, drug release is more dependent on the swelling kinetics of the hydrogel mediated by the environment pH changes.
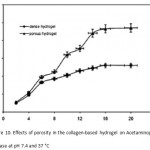 |
Figure 10: Effects of porosity in the collagen-based hydrogel on Acetaminophen release at pH 7.4 and 37 °C. |
It shows the pH triggered collapse and resultant burst release due to squeezing effect.The dependence of the extent of crosslinking on in vitro release was also displayed in Fig. 11. It is observed that release rates depend upon the amount of MBA used as crosslinking agent. The cumulative drug release of Acetaminophen from the hydrogels was decreased with increasing MBA content 7. This could be due to the fact that at higher crosslinking, free volume of the matrix will decrease, thereby hindering the transport of drug molecules through the matrix.
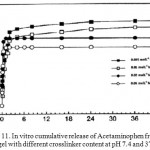 |
Figure 11: In vitrocumulative release of Acetaminophen from the hydrogel with different crosslinker content at pH 7.4 and 37°C. |
Conclusion
A novel protein-based superabsorbent hydrogel was synthesized via graft copolymerization of 2-acrylamido-2-methylpropanesulfonic acid (AMPS) onto collagen backbones in an aqueous solution using a persulfate initiator and a hydrophilic crosslinker. the superabsorbent hydrogels exhibited high sensitivity to pH, so that, several swelling changes of the hydrogel were observed in pH variations of a wide range (1-13). Ionic repulsion between charged groups incorporated in the gel matrix by an external pH modulation could be assumed as the main driving force responsible for such abrupt swelling changes. Furthermore, the reversible swelling-deswelling behavior in solutions with acidic and basic pH makes the hydrogels a suitable candidate for controlled drug delivery systems.
Our results indicated that the porous hydrogel, prepared by the gas foaming technique, could potentially be used as a carrier for local and controlled delivery of drugs.the release value of Acetaminophen from hydrogels at pH 7.4 was higher than that at pH 1.2 due to the electrostatic repulsion between carboxylate groups. finally, These results suggest that a porous hydrogel could potentially be a useful local delivery system to release drugs primarily at a specific site of body.
Refrerences
- Eddington, D.T., Beebe, D.J. Flow control with hydrogels. Adv. Drug Deliv.Rev. 56: 199-210 (2004).
- Hamidi, M., Azadi, A., Raûei, P.Adv. Drug Deliv.Rev. 60: 1638-1649 (2008).
- Koo, H., Jin, G., Kang, H., Lee, Y., Nam, H.Y., Jang, H., Park, G.S. International Journal of Pharmaceutics. 374: 58-65 (2009).
- Kakinoki, S., Taguchi, T., Saito, H., Tanaka, J., Tateishi, T.Eur. J. Pharm. Bio. 66: 383-90 (2007).
- Kim, S.J., Spinks, G.M., Prosser, S., Whitten, P.G., Wallace, G.G., Kim, S.I.,Nat. Mater. 5: 48-51 (2006).
- Kranz, H., Bodmeier, R. Eur. J. Pharm. Sci. 34: 164-72 (2008).
- Sadeghi,M, Yarahmadi, M, Oriental Journal of Chemistry, 27(2): 417-427 (2011)
- Sadeghi,M, Yarahmadi, M, Oriental Journal of Chemistry, 27(2): 417-427 (2011)
- Sadeghi,M, Yarahmadi, M, Journal of Chemistry, 27(2): 453-460 (2011).
- Kwon, I.C., Bae, Y.H., Kim, S.W.Nature, 354: 291-293 (1991).
- Oh, J.K., Drumright, R., Siegwart, D.J., Matyjaszewski, K.Prog. Polym.Sci. 33: 448-77 (2008).
- Siepmann, J., Peppas, N.A. Adv. Drug Deliv. Rev. 48: 139-57 (2001).
- Soppimath, K.S., Aminabhavi, T.M., Dave, A.M., Kumbar, S.G., Rudzinski, W.E. Drug Dev. Ind. Pharm. 28: 957-974 (2002).
- Tatsuma, T., Takada, K., Miyazaki, T.Adv. Mater.19: 1249-1251 (2007).
- Thornton, P.D., Mart, R.J., Ulijn, R.V.Adv. Mater.19: 1252-1256 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.





