How to Cite | Publication History | PlumX Article Matrix
Effect of Trichoderma species on Pythium aphanidermatum Causing Rhizome Rot of Turmeric
N. Uma Maheswari* and T. P. Sirchabai
PG and Research Department of Microbiology, Sengamala Thayaar Educational Trust Women’s College, Mannargudi - 614 001 India.
ABSTRACT: Turmeric (Curcuma longa) belonging to the family Zingiberaceae is one of the most ancient and importance spice crop grown in India. Rhizome rot of turmeric, caused by Pythium aphanidermatum (Edson) Fitz., is a major constraint for the production of healthy rhizome, some times causing total failure crop. Soil samples was collected and analyzed for physico chemical parameters, then the effect of pH, N, P and K also studied. Maximum growth the pathogen was noticed in pH 6 (55mm), 2% of nitrogen (21mm), 1% of phosphorus (16mm) and 2% of potash (14mm). Pythium aphanidermatum against chemical fungicide thiophanate methyl 70% WP produced inhibition zone (23mm) of fungal growth in disc diffusion assay. Because of the inherent risks associated with the chemical control, an attempt has been made in present study to control the rhizome rot by biological means under laboratory method. In vitro studies on biocontrol agents against turmeric rhizome rot pathogen Pythium aphanidermatum revealed that fungal bioagent was inhibiting the growth of pathogen. Trichoderma viride (79mm) was best inhibiting the colony growth of Pythium aphanidermatum (11mm) in 288 hours P = <0.001 statistically significant difference. Experts recommend that rhizome treatment with biofungicide like Trichoderma spp., can effectively control of this disease.
KEYWORDS: Pythium aphanidermatum; Turmeric; Trichoderma viride; Biofungicide; Rhizome rot
Download this article as:| Copy the following to cite this article: Maheswari N. U, Sirchabai T. P. Effect of Trichoderma species on Pythium aphanidermatum Causing Rhizome Rot of Turmeric. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Maheswari N. U, Sirchabai T. P. Effect of Trichoderma species on Pythium aphanidermatum Causing Rhizome Rot of Turmeric. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9529/ |
Introduction
Turmeric (Curcuma longa.,L) is an important condiment and a useful dye, with varied uses in drug and cosmetic industries. It is used medicinally for external application and taken internally as a stimulant. “Kum-Kum”, popular for every house wife, is also a byproduct of turmeric. Turmeric is known as the “Golden spice” and has been used in India as a medicinal plant, and held sacred from time immemorial. It is used commercially in pharmaceuticals, confectionary, food and cosmetic industries. It exhibits various medicinal properties which include germicidal, anti-tumour, anti-bacterial, anti-protozoal, anti-helmentic, anti-inflammatory, antioxidant and cholesterol lowering properties. (Kandiannan et al., 2008).
Turmeric being a Zingiberaceous species, has disease problems similar to that of ginger (Dohroo, 2005). There are diseases that affect rhizomes, such as the rhizome rot, which kills the plant, and those affecting the aerial shoot, such as leaf blotch and leaf spots, which lead to severe reduction in yield. These diseases occur in all turmeric growing regions, but their occurrence and severity very much, depending upon the growing region and growing condition. Among the diseases affecting turmeric, rhizome rot and foliar diseases are the most serious. Turmeric is propagated exclusively through underground rhizomes. Since rhizome multiplication is slow, maintenance of planting material is expensive. Furthermore, the highly susceptibility of this crop to various diseases and pests is a major constrains in the production of turmeric (Sarma, and Anandaraj, 2000).
Pythium soft rot of turmeric is notoriously difficult to manage (Kamoun et al., 1999). Chemical treatment of seed rhizome and application of biocontrol agents have been used to control soft rot. However, genetic improvement, the most desirable method of disease management, has been so far limited in turmeric for two reasons. First, all turmeric cultivars available today are highly susceptible to soft rot and no resistance or tolerant source has yet been identified. Second since turmeric is an obligatory asexual species, propagated exclusively through its rhizomes, gene introgression through sexual crossing is impossible. Consequently, no effort has been made to systematically evaluate the wild relatives of turmeric for soft rot resistance.
Trichoderma is a potent biocontrol agent and used extensively for Post-harvest disease control. Major diseases of turmeric are rhizome rot and wilts. Experts recommend that rhizome treatment with biofungcide like Trichoderma spp., can effectively control this disease(Howell, 2004). The objective of this study was to investigate the effect of Trichoderma spp on Pythium aphanidermatum causing rhizome rot of turmeric.
Materials and Methods
Isolation and Identification of pathogen (Barnett, 1972 and Gilman, 1957 )
The soil sample was collected from cultivable land of Thethuvasal patti village, Pudukkottai district, Tamilnadu. From the soil sample, the Pythium aphanidermatum was isolated by serial dilution technique. The morphological characteristics were identified by lactophenol cotton blue staining.
Physico chemical parameters of soil
Moisture content of the soil sample was suspended in distilled water (1:2 w/v) and allowed to settle down the sand particles. The pH of the suspension was recorded using digital meter. Electrical conductivity of soil was determined in the filtrate of the water extract using Conductivity Bridge as described by Jackson (1973), and cation exchange capacity (CEC) of the soil was determined by using 1N ammonium acetate solution as described by Jackson (1973). Organic carbon content of the soil was determined by adopting chromic acid wet digestion method of described by Walkey and Black (1934), available nitrogen was estimated by alkaline permanganate method as described by Subbaiah and Asija (1956), and available phosphorous by Bray’s method as described by Bray and Kutz (1945). Available potassium was extracted from soil with neutral 1N ammonium acetate (1:5) and the potassium content in the extract was determined by using flame photometer (Standfold and English, 1949). Calcium (Neutral 1 NNH4 OAC extractable 1:5) was extracted with neutral 1N ammonium acetate and the available calcium in the extract was determined by versenate method (Jackson, 1973).
Pathogenicity test (pot culture method) (Ghoneim et al., 1996)
Pathogen was isolated from infected turmeric roots and rhizomes and barely inoculums were prepared. Then 10mm agar discs were cut with potato dextrose agar culture of Pythium aphanidermatum was inoculated in barely containing flask. The inoculum was incubated at 28±2°C for 7days. The turmeric seeds were surface sterilized and pregerminated in sterile vermiculture for fifteen days at 30±2°C in a growth cabinet. Barely seed inoculums of Pythium aphanidermatum was mixed in to steam pasteurized soil and fulfill the pots (30g/kg). On the other hand sterile barely seeds were mixed in pasteurized soil in same rate. Turmeric seedling was transplanted into each pot.
Effect of pH (Aneja, 2002)
The Potato Dextrose agar medium was prepared in various pH concentrations like 4, 5, 6, 7, and 8 the medium was poured in to the sterile Petri dishes in triplicate form separately. The pathogen 6mm diameter was inoculated on the different pH of PDA medium from already stored pure culture of Pythium aphanidermatum. After incubation period, the plates were incubated at 270 ±20c for 72hours. The growth was measured and results were recorded.
Effect of N, P, K (Jagadeeswaran et al., 2005)
The PDA medium was prepared in various concentrations of N, P and K separately. The concentration of N P K was 1, 2 and 3 percentage. The medium was poured into the sterile Petri dishes in triplicate form separately. The pathogen 6mm diameter was inoculated on the different concentrations of N, P and K altered PDA medium from already stored pure culture of Pythium aphanidermatum. After incubation period, the plates were incubated at 270 ± 20c for 72hours.
Antagonistic activity (Dennis and Webster, 1971)
The bio control agent Trichoderma viride was selected to study the antagonistic activity against Pythium spp isolated from test sample. The PDA medium was prepared and poured in to the Petriplate. After solidification pure culture of Trichoderma viride and Pythium spp., was placed on the medium in opposite direction. The plates were incubated at 27± 20 C for 15 days and the results were noted at every 72 hours on 3, 6, 9, 12 and 15th days respectively.
Disc diffusion method (Kirby bauer, 1961)
The PDA medium was prepared and sterilized at 1210 C for 15 minutes and allow it to cool approximately 500C. Then the medium was poured into the sterile Petriplate. After solidification the isolated pathogen Pythium aphanidermatum was swabbed on the agar plate with help of sterile cotton buds. Disc preparation was done by using thiophanate methyl 70% WP.
Statistical analysis
All the experiments were performed in triplicate (n= 3). Data are presented as mean ± SEM. Data were analysed using one-way analysis of variance test (ANOVA). Values of P≤ 0.05 were selected as showing a statistically significant difference (SAS Institute, 1996).
Result
Isolation and identification of fungus from turmeric field
Infected turmeric soil sample was collected and inoculated on PDA medium and incubate at 27°C for 72 hours. After incubation period the plates were examined fungal colonies were observed and identified using by lactophenol cotton blue staining method. The following organisms were identified from sample, Aspergillus awamori, Aspergillus conicus, , Aspergillus luchensis and Pythium aphanidermatum.
Physico chemical parameters of soil
The soil pH was 7.20. The available nitrogen, phosphorus, potash content of the soil sample such as 102.3 kg/ac, 64.8 kg/ ac, 142kg/ac (Table-1).
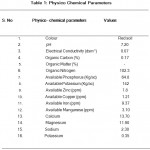 |
Table 1: Physico Chemical Parameters.
|
Pathogenicity test
In pathogenicity test, two treatments were carried out one is control and treatment. In pot culture experiment plants were grown, control plants were not infected but treatment plants were infected by pathogen and the symptoms were observed. In plate culture method the Pythium aphanidermatum organism was observed in PDA plate where the sample taken from disease infected pots.
Effect of pH
The Pythium aphanidermatum maximum growth was noted in PDA plate at pH6 compared with other pH-5, 7 and 4. In pH8 the fungal growth was gradually decreased. The colony diameter was followed respectively, 55mm, 52mm, 45mm, 41mm and 37mm. (Table-2).
Effect of N, P, K
In PDA medium the Pythium aphanidermatum was growth in different concentration of NPK was recorded as followed. In effect of Nitrogen 1% – 16mm, 2% – 21mm and 3% – 18mm. In phosphorus 1% – 16mm, 2% -17mm and 3% – 13mm , effect of potash 1% – 14mm, 2% – 13mm and 3% – 11mm were recorded. (Table-2).
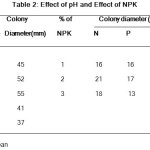 |
Table 2: Effect of pH and Effect of NPK.
|
Antagonistic activity
Trichoderma viride grew quickly and dominate the Pythium spp with in 15 days. After 21 days the pathogen was completely inhibited by antagonist T.viride. The mycelium growth rate of Trichoderma viride and Pythium spp respectively, 25mm, 18mm on 3rd day 56mm, 28mm on 9th day, 63mm, 27mm on 12th day. The T.viride grew fast compared with pathogen on 72 hours 25mm. Data were analysed using one-way analysis of variance test at 288 hours P = <0.001 vs P.aphanidermatum and the 72 hours P = 0.015 Vs 288 hours P.aphanidermatum. The difference in the mean values of the two groups is greater than would be expected by chance; there is a statistically significant difference between the input groups (Table-3).
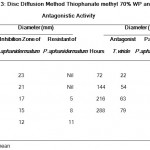 |
Table 3: Disc Diffusion Method Thiophanate methyl 70% WP and Antagonistic Activity.
|
Disc diffusion
After incubation period the plates were examined the pathogen Pythium spp inhibited by disc diffusion method and the disc was prepared by commercially available systemic fungicide Thiophanate methyl 70% WP. The 24mm clear zone was observed in 72 hours, the zone maintain till 120 hours but the pathogen tolerate the chemical fungicide and grew on zone respectively 5mm on 168 hours, 7mm on 216 hours and 9mm on 264 hours. The chemical fungicide inhibits the growth of Pythium spp and form clear zone up to 23mm at 72 hours. Pythium spp was able to develop resistance and grow well against chemical fungicide after 264 hours. Data were analysed using one-way analysis of variance test at 72 hours P = 0.002 Vs 264 hours inhibitory zone and the 72 hours P = 0.001 Vs 264 hours resistance zone. The difference in the mean values of the two groups is greater than would be expected by chance; there is a statistically significant difference between the input groups (Table-3).
Discussion
One species adversely affects the environment for another species is known as antagonism. Such organisms may be of great practical importance. Since they produce antibiotics or other inhibitory, substances which affect the normal growth processes or survival other organisms. Antagonistics relationships are quite common in nature Pelczar et al., (1996). The same phenomenon was also observed in our present study also.
In our finding similar to soil physio chemical parameters were analyzed and then the effect of pH and N, P, K were studied. The pathogen was maximum growth in pH 6 and 2% of nitrogen, 1% of potash and 2% of phosphorous Bray and Kutz (1945). Rhizome rot is the major devastating disease in turmeric with a yield reduction of 40- 60%. The disease caused by a fungus Pythium spp., it spreads through affected rhizomes and infect soil. Similar reported by Rajendran et al., (2010) T.viride inhibited the growth of pathogens such as A.niger, A.flavus, A.fumigatus, Fusarium spp and Penecillium spp in dual culture, this result is identifiably to that of our work. We also prove antagonistic activity of Trichoderma viride was reported by Rama Bhandra Raju et al., (2000) is deviated from the present study. According to their reports Trichoderma viride produce inhibition zone of 9mm, against pathogen, but we got a zone of inhibition of 23-79mm. Trichoderma effectively control Pythium species. William and Asher (1996) reported that the type of antifungal metabolite produced by the isolates may vary.
Conclusion
Turmeric is one of the most economic spices crops of India. It suffers from several serious diseases. One among them is the rhizome rot disease or root rot disease caused by Pythium Spp. This disease is prevalent in this part of our state too. Normally chemical control of the disease is achieved by spraying suitable fungicide. But because of the inherent risks associated with the chemical control, an attempt has been made in the present study to control the rhizome rot by biological means under field conditions. The work in enhancing the biocontrol ability of microbes through mutation (UV or gamma radiation), molecular biology, protoplast fusion and other biotechnological methods needs to be pursued further.
References
- Aneja, K.R. Experiments in Microbiology Plant pathology and Biotechnology, 4th Eds. New age International Publishers, New Delhi. 119-228. (2002).
- Barnett, H.L. Illustrated genera of imperfect fungi, 3rd Eds. Burgess Publishing company. California. 435-460. (1972).
- Bray, R.H. and Kutz, L.T. Determination of total organic and available phosphorus in soils. Soil Sci., 59: 39-45. (1945).
- Dennis, C. and Webster, J. Antagonistic properties of species groups of Trichoderma in production of non-volatile antibiotics. Brit. Mycol Soc. 57: 25-39. (1971).
- Dohroo, N.P. Diseases of ginger. In: Ravidram, P.N. and Nirmal Babu, K. (eds.) Ginger the genus Zingiber. Boca Raton, Florida, U.S.A.: CRC Press. 305–340. (2005).
- Ghoneim, S.S.H., Abdel-Massih, M.I. and Mahmoud, F.F.A. Interaction between root-knot nematode and root rot on Olive trees. Annals Agric. Sci., Ain Shams Univ. 41: 445-461. (1996).
- Gilman, J.C. A manual of soil fungi, 2nd Eds. Oxford and IBH Publishing company, Calcatta. 117-128. (1957).
- Howell, C.R. Understanding the mechanisms employed by Trichoderma virens to affect biological control. Phyto pathology. 94: S138 (supplement). (2004).
- Jackson, M.L. Soil chemical analysis. Prentice hall of India Pvt. Ltd., New Delhi. 125-140. (1973).
- Jagadeeswaran, R. Murugappan, V. and Govindaswamy, M. Effect of slow release NPK fertilizer sources on the nutrient use efficiency in turmeric (Curcuma longa L.). World J. Agric. Sci. 1(1): 65-69. (2005).
- Kandiannan, K., Sasikumar, B., Suseela Bhai, R., Santhosh, C.K., Eapen, J., Devasahayam, S. and John Zachariah, T. Rhizome rot of Turmeric. Indian Institute of Spices Research. Calicut., Kerala. 8-30. (2008).
- Kamoun, S., Huitema, E. and Vleeshouwers, V.G.A.A. Resistance to Oomycetes: a general role for the hypersensitive response. Trends in Plant Science. 4: 196-200. (1999).
- Kirby Bauer, A.W., Kirby, W.M.M., Serris, J.C. and Turek, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. clinic. Pathol. 45: 493-496. (1996).
- Pelczar, J.R. Microbiology. Published by TaTa McGRAW-HILL and 5th Eds and printed at SDR printers, West Jyothi Nagar, Delhi. 543-568. (1996).
- Rama Bhandra Raju, M. and Krishna Murthy, K.V.M. Efficacy of Trichoderma sp in the management of collar rot of groundnut caused by Aspergellius niger van Teighem. Indian J. Plant prot. 28: 197 – 199. (2000).
- Rajendran, R., Jegadeeshkumar, D., Sureshkumar, B.J. and Nisha, T. Invitro assessment of antagonistic activity of Trichoderma viride against post harvest pathogens. J. Agri technol. 6: 31 – 35. (2010).
- SAS Institute. SAS / STAT User’s Guide, Version 6, 12th Eds. 846 pp. SAS Institute, Inc. Cary, North Carolina. (1996).
- Sarma, Y.R. and Anandaraj, M. “Disease of spice crops and their management”. Ind.J. Arecanut, Spices and Medicinal Plant., 2(1): 6-20. (2000).
- Standfold, S. and English, L. Use of flame photometer I rapid soil test for K and Ca. Journal of Agronomy. 41: 419-428. (1949).
- Subbaih, B.V. and Asija, G.L. A rapid method for estimation of available nitrogen in soil. Curr. Sci. 25: 258-260. (1956).
- Walkey, A. and Black, I.A. Rapid titration method. Soil Sci. 37: 29-38. (1934).
- Williams, G.E. and Asher, M.J.C. Selection of Rhizobacteria for the control of Pythium ultimum and Aphanomyces cochlioides on sugar-beet seedlings. Crop prot. 15: 479: 486. (1996).

This work is licensed under a Creative Commons Attribution 4.0 International License.





