How to Cite | Publication History | PlumX Article Matrix
S. Ganguly* and A. K. Banik
Department of Chemical Engineering, Biochemical Engineering Division, Biotechnology laboratory, University of Calcutta, Kolkata - 700 009 India.
Corresponding Author E-mail: subhadeepgangulyphysiol@rediffmail.com
ABSTRACT: An experimental study was carried out to investigate the effect of vitamin B-Complex on growth and l-glutamic acid accumulation by an auxotrophic mutant Micrococcus glutamicus AB100. Different concentrations (0.1 – 2.0 µg/ml) vitamin B12, Folic acid thiamine-HCl, riboflavin, nicotinic acid, pyridoxine-HCl, inositol, calcium pantothanate, paraamino-benzoic acid and biotin were examined. Production was maximum with vitamin B12, 1.0 µg/ml; Folic acid, 0.5 µg/ml; thiamine-HCl, 1.0 µg/ml; riboflavin, 0.5 µg/ml; nicotinic acid, 0.5 µg/ml; pyridoxine-HCl, 0.7 µg/ml; inositol, 0.6 µg/ml; calcium pantothanate, 0.4 µg/ml; paraamino-benzoic acid 0.3 µg/ml; and biotin 0.2 µg/ml. Production was decreased with further increment of the vitamin concentrations, but dry cell weight increased continuously with increased levels of the vitamins.
KEYWORDS: Mutant; Micrococcus glutamicus; Vitamin; Dry cell weight
Download this article as:| Copy the following to cite this article: Ganguly S, Banik A. K. Effect of Vitamin B-complex on Growth and L-glutamic acid Accumulation by a Mutant Micrococcus glutamicus AB100. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Ganguly S, Banik A. K. Effect of Vitamin B-complex on Growth and L-glutamic acid Accumulation by a Mutant Micrococcus glutamicus AB100. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9609 |
Introduction
Fermentative production of l-glutamic acid has been used during last fifty years and the production yield has been increased significantly over the years [1-3]. Different nutrients parameters also known to significantly alter the product yield.
Many microorganisms used for the production of l-amino acids including l-glutamic acid required different vitamins for their growth and metabolites accumulation. Several reviews are available on the requirements of vitamin B-Complex for the microbial production of l-amino acids [4-15].
Considering all these reviews, the present study was intended to study the effect of vitamin B-complex on growth and l-glutamic acid accumulation.
Materials and Methods
Microorganism
Micrococcus glutamicus AB100, a biotin requiring auxotrophic mutant derived from a regulatory mutant Micrococcus glutamicus AB1 by induced mutation in our laboratory was used throughout the study [16].
Synthetic medium used for l-glutamic acid production
The composition of the synthetic medium used for L-glutamic acid production was as follows : glucose, 9.0%; diammonium hydrogen phosphate, 1.4%; dipotassium hydrogen phosphate, 0.15%; magnesium sulfate, hepta hydrate; 0.03%; calcium carbonate, 0.04; ferrous sulfate, hepta hydrate, 5.0 µg/ml; zinc sulfate, hepta hydrate, 1.0 µg/ml; manganese sulfate, tetrahydrate, 1.0 µg/ml; and biotin, 0.2 µg/ml; pH 6.5.
Fermentation was carried out using shake, flask method on a rotary shaker (150 rpm) in 100 ml Erlenmayer conical flask containing 20 ml mineral salt medium for 72h at 29oC. The medium was inoculated with 4.0% (v/v) of 48h old seed culture (6.0 X 104 cells) of Micrococcus glutamicus AB100 [17].
Addition of vitamin B-complex to the synthetic medium
Initially, the basal medium contained only biotin (0.2 µg/ml) as a member of vitamin B-complex. Different members of vitamin B-complex namely vitamin B12, folic acid, thiamine-HCl, riboflavin, nicotinic acid, pyridoxine-HCl, inositol, biotin, Calcium pantothanate, paraaminobenzoic acid and biotin were added separately to the medium at varying concentrations (0.1-2.0 µg/ml) [15].
Analysis of Amino acid
Descending paper chromatography was used for detecting l-glutamic acid in culture medium and was run for 18h on a Whatman no. 1 chromatography paper solvent system used include n-butanol : acetic acid : water (2 : 1 : 1). The spots were visualized by spraying with a solution of 0.2% ninhydrin in acetone and quantitative estimation of l-glutamic acid in the suspension was done using colorimetric estimation method [18,19].
Estimation of Dry Cell Weight
After centrifugation, a few ml of 1.0 (M) HCl was poured into the precipitate of the bacterial cells and calcium carbonate to dissolve calcium carbonate to dissolve calcium carbonate. The remaining bacterial cells were washed with water and derived at 100oC until cells weight remain constant [12].
Statistical Analysis
All data were expressed as mean ± SEM, where n = 6. The data were analyzed by one way ANOVA followed by Dunett’s post-hoc multiple comparison test using “prism 4.0” software (Graph pad Ind., USA). A “p” value less than 0.05 was considered significant and less than 0.01 as a highly significant.
Result and Discussion
Fig. 1 – 10 showed the effect of different members of vitamin B-complex on growth and l-glutamic acid production by the mutant Micrococcus glutamicus AB100. All the vitamins studied showed positive effect on growth and the production. Maximum l-glutamic acid production was obtained with vitamin B12, 1.0 µg/ml; Folic acid, 0.5 µg/ml; thiamine-HCl, 1.0 µg/ml; riboflavin, 0.5 µg/ml; nicotinic acid, 0.5 µg/ml; pyridoxine-HCl, 0.7 µg/ml; inositol, 0.6 µg/ml; calcium pantothanate, 0.4 µg/ml; paraamino-benzoic acid 0.3 µg/ml; and biotin 0.2 µg/ml. Production of l-glutamic acid was decreased significantly (p<0.01) after omittion of biotin from the fermentation medium. Dry cell weight was increased continuously with the increment of the vitamin concentrations. Production of l-glutamic acid was decreased significantly (p<0.05 and p<0.01 respectively) from 0.4-2.0 µg/ml concentration of biotin.
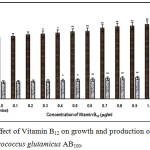 |
Figure 1: Effect of Vitamin B12 on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
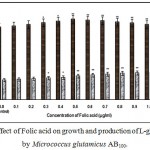 |
Figure 2: Effect of Folic acid on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
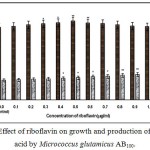 |
Figure 3: Effect of thiamine-HCl on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
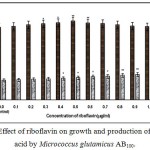 |
Figure 4: Effect of riboflavin on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
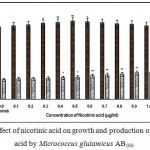 |
Figure 5: Effect of nicotinic acid on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
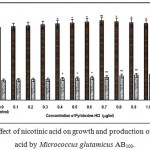 |
Figure 6: Effect of nicotinic acid on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
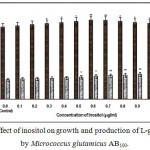 |
Figure 7: Effect of inositol on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
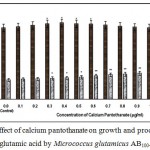 |
Figure 8: Effect of calcium pantothanate on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
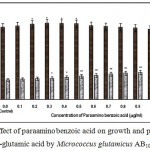 |
Figure 9: Effect of paraamino benzoic acid on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
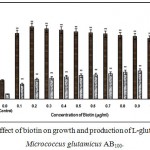 |
Figure 10: Effect of biotin on growth and production of L-glutamic acid by Micrococcus glutamicus AB100. |
(Values were expressed as mean ± SEM, where n = 6; *p<0.05, **p<0.01 when compared to control)
Biotin was suggested as a co-factor for glucose oxidation, protein synthesis, cellular permeabilihy and co-valent bond formation with cobalt [20]. Lactic acid accumulation was increased with rining level of biotin due to excess cellular population which created anaerobic condition, and at higher biotin concentration, lactic acid should be treated as the main fermentation product, accompanied by low levels of succinic acid and malic acid [1]. Takahashi et al (1965) reported that among different vitamins tested, thiamine-HCl significantly stimulated l-glutamic acid production by a newly isolated strain (S10B1) of corynebacterium [21]. Ekwealor and obeta (2007) studies the effect of thiamine-HCl, nicotinic acid, biotin, pyridoxine-HCl, folic acid and riboflavin on l-lysine production by Bacillus sp and reported that biotin was essential for l-lysine production by this strain [15].
However, in our present investigation, all the vitamins examined simulated the growth and l-glutamic acid production upto certain concentrations which were depicted in Fig. 1 – 10, but higher concentrations of vitamins simulated the growth, but production was decreased gradually, probably due to anaerobic conditions created by ecess cellular population.
Reference
- Kinoshita S, Vdaka S and Shimono M, studies on the amino acid fermentation. Part I. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol, 3, 1957, 193.
- Chao K C and Foster J W, A glutamic acid producing Bacillus. J. Bacteriol, 77 : 1959 : 715-725.
- Kim HS and Ryu DDY, continuous glutamate production using an immobilized whole cell system, Biotechnol. Bioeng, 24, 1982, 2167-2174.
- Okumura S, Tsugawa R and Tsunoda T, Studies on the l-glutamic acid fermentation. Part II. Activity of L-biotin and biotin group compounds as a growth factor, Agric chem.. Soc. Jpn, 36, 1962, 197-203.
- Tanka K, Iwasaki T and Kinoshita S, studies on L-glutamic acid fermentation. Part V. Biotin and L-glutamic acid accumulation by bacteria, Agric. Chem. Soc. Jpn, 34, 1960, 593-600.
- Tauro P, Ramachandra Rao T N, Johar D S and Srinivas A, Studies on microbial production of glutamic acid, food sci, 17, 1963, 263-266.
- Birnbaum J and Demain A L, Reversal by citrate of the idoacetate and fluoride inhibition of glutamic acid production by corynebacterium glutamicum, Microbiol, 18(2), 1969, 287-288.
- Nunheimer T D, Birnbaum J, Ihnem E D and Demain A L, Product inhibition of the fermentative formation of Glutamic Acid, Appl. Microbiol, 20(2), 1970, 215-217.
- Ward O P, Fermentation raw materials, Fermentation Biotechnology : principles, processes and products, practice Hall, 1989, 59-71.
- Yamada S, Nabe K, Ujimaru T, Izuo N and Chibata I, Extracellular accumulation of a new amino acid, 0-2-hydroxypropylhomoserine, from 1,2-propanediol by Flavobacterium rigense, Environ, Microbiol, 35, 1978, 1046-1051.
- Shah A H, Hameed A and Khan G M, Improved Microbial production of lysine by Developing a new Auxotrophic mutant of Corynebacterium glutamicum J. Biol Sci, 5(1), 2002, 80-83.
- Shah A H, Hameed A and Khan G M, Optimization of culture conditions for L-lysine fermentation by corynebacterium glutamicum, on line J. Biol Sci, 2(3),2002, 151-156.
- Yugandhar N M, Kiran Babu U, Lalitha K, Jaya Raju K and Raddy D S R, Production of glutamic acid using Breviacterium roseum with free and immobilized cells, Res, J. Microbiol, 2(7), 2007, 584-589.
- Yugandhar N M, Kiran Babu U, Raju Ch A I, Raju J and Raddy D S R, Optimization of Glutamic acid production by Breviacterium roseum, Res. J. Microbiol, 1(5), 2006, 428-432.
- Ekwealor I A and Obeta J A N, Effect of vitamins and bivalent metals on lysine yield in Bacillus megaterium, J. Biotechnol, 6(11), 2007, 1348-1351.
- Ganguly S and Banik A K, Induced mutation and selecetion of High yielding strain of Micrococcus glutamicus for glutamic acid production, J. Indian Chem. Soc, 87(6), 2010, 711-721.
- Ganguly S and Banik A K, Optimization of physical conditions for the production of l-glutamic acid by a mutant Micrococcus glutamicus AB100, Int. J. Pharm. Biol Sci, 2(2), 2011, B-295-299.
- Chinard F P, Photomeric estimation of proline and ornithine, J. Biol. Chem, 199, 1952, 91-95.
- Spies J R, Colorimetric procedures for amino acids, Methods in Enzymol, 3, 1957, 468-471.
- Oishi K and Aida K, Studies on amino acid fermentation XI. Effect of biotin on the Embden Meyerhof-Parnas Pathway and the hexose monophosphate shunt in a glutamic acid producing bacterium, Brevibacterium ammoniagenes, Agric, Biol, Chem, 29, 1965, 83-89.
- Takahashi J, Kobayashi K, Imada Y and ymada K, Effects of Corn Steep Liquor and Thiamine on L-glutamic Acid fermentation of Hydrocarbons. IV. Utilization of hydrocarbons by Microorganisms, Appl, Microbiol, 13(1), 1965, 1-4.

This work is licensed under a Creative Commons Attribution 4.0 International License.





